Home /
Expert Answers /
Chemistry /
yttrium-90-is-a-radioactive-isotope-used-to-treat-tumors-in-the-liver-90-y-has-a-half-life-of-2-pa188
(Solved): Yttrium-90 is a radioactive isotope used to treat tumors in the liver. ^(90)Y has a half-life of 2. ...
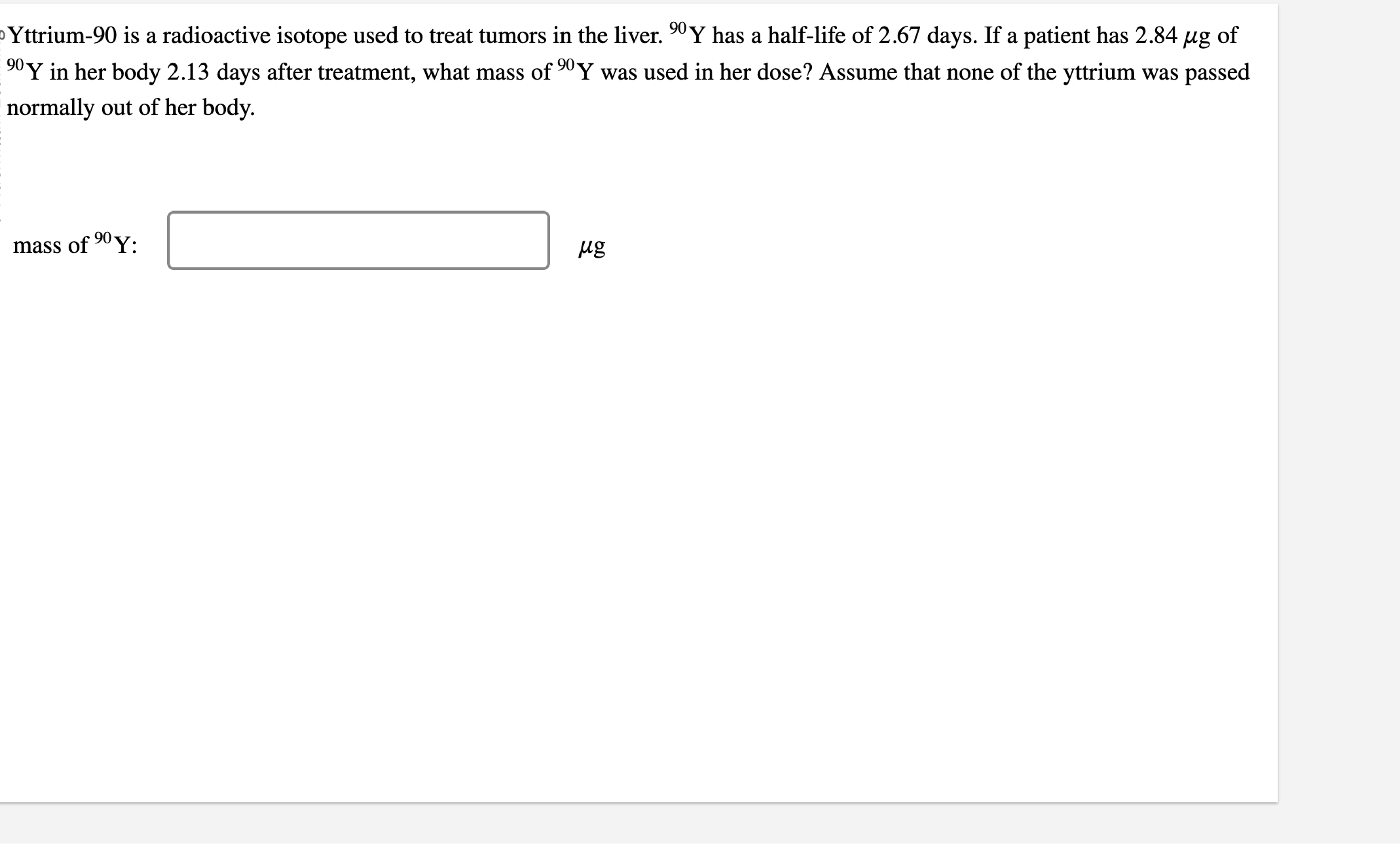
Yttrium-90 is a radioactive isotope used to treat tumors in the liver.
^(90)Yhas a half-life of 2.67 days. If a patient has
2.84\mu gof
^(90)Yin her body 2.13 days after treatment, what mass of
^(90)Ywas used in her dose? Assume that none of the yttrium was passed normally out of her body. mass of
^(90)Y: