Home /
Expert Answers /
Chemistry /
xercise-resonance-aromatic-substitution-the-carbocation-formed-in-para-nitration-has-three-resonan-pa504
(Solved): XERCISE Resonance: Aromatic Substitution The carbocation formed in para nitration has three resonan ...
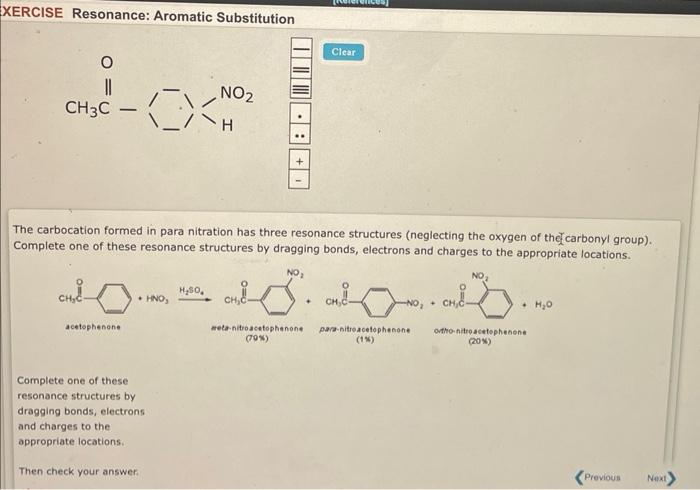
XERCISE Resonance: Aromatic Substitution The carbocation formed in para nitration has three resonance structures (neglecting the oxygen of the?? carbonyl group). Complete one of these resonance structures by dragging bonds, electrons and charges to the appropriate locations. Complete one of these resonance structures by dragging bonds, electrons and charges to the appropriate locations. Then check your answer:
Expert Answer
Nitration is an electrophilic aromatic substitution.In step 1, Benzene ring attacks the electrophile (Nitronium ion)