Home /
Expert Answers /
Chemistry /
write-the-equilibrium-constant-expression-for-the-reaction-shown-below-2-mathrm-n-2-mathrm-pa846
(Solved): Write the equilibrium constant expression for the reaction shown below \[ 2 \mathrm{~N}_{2} \mathrm ...
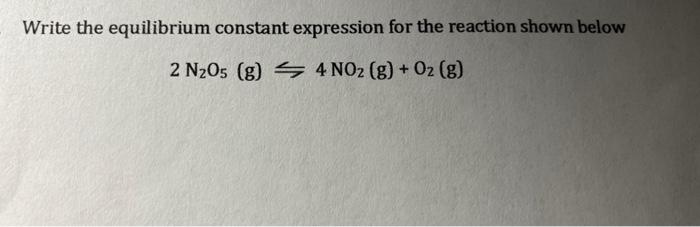
Write the equilibrium constant expression for the reaction shown below \[ 2 \mathrm{~N}_{2} \mathrm{O}_{5}(\mathrm{~g}) \leftrightharpoons 4 \mathrm{NO}_{2}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g}) \]
Expert Answer
Given chemical equation: 2N2O5(g)?4NO2(g)+O2(g) Since, equilibrium constant is the ratio of active masses of