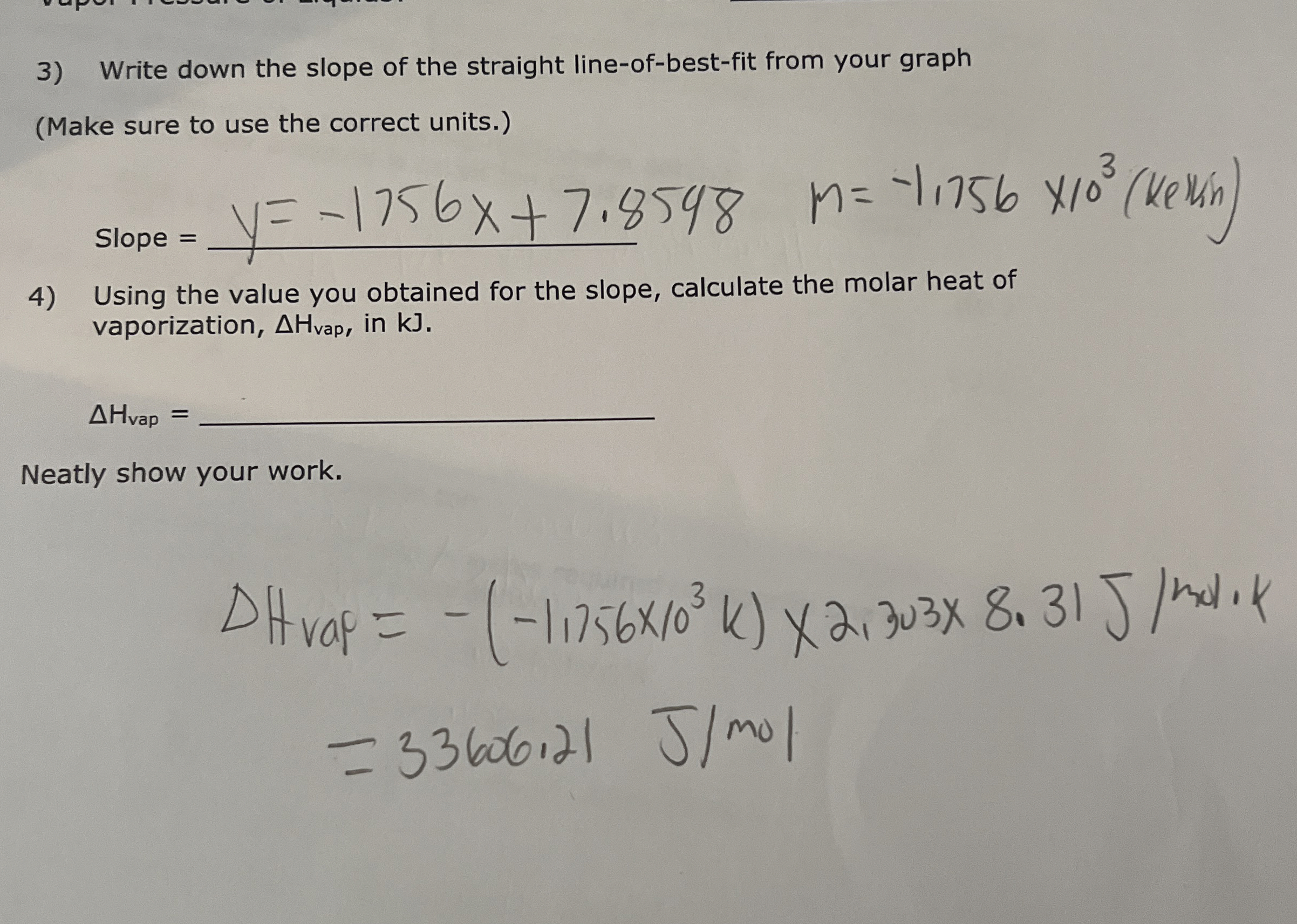Home /
Expert Answers /
Chemistry /
write-down-the-slope-of-the-straight-line-of-best-fit-from-your-graph-make-sure-to-use-the-correct-pa425
(Solved): Write down the slope of the straight line-of-best-fit from your graph (Make sure to use the correct ...
Write down the slope of the straight line-of-best-fit from your graph (Make sure to use the correct units.) Slope
=
q,
q,
n=-1,756\times 10^(3) (Kelun ) Using the value you obtained for the slope, calculate the molar heat of vaporization,
\Delta H_(vap ), in kJ .
\Delta H_(vap )=
q,Neatly show your work.
\Delta H_(rap )=-(-1.1756\times 10^(3)(K))\times 2,303\times 8.31(J)/(m)od.k
=33606,21(J)/(m)ol