Home /
Expert Answers /
Chemistry /
why-are-some-solutes-soluble-in-water-and-some-solutes-soluble-in-cyclohexane-0-1-mathrm-m-pa635
(Solved): Why are some solutes soluble in water and some solutes soluble in cyclohexane? \( 0.1 \mathrm{M} \ ...
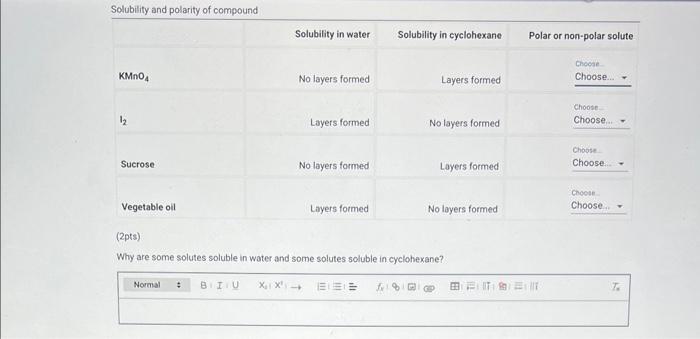
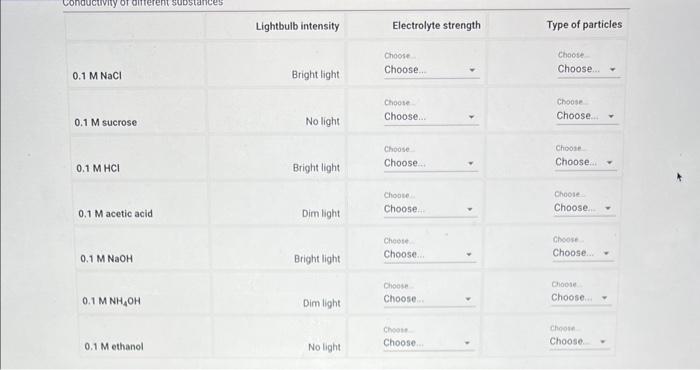

Why are some solutes soluble in water and some solutes soluble in cyclohexane?
\( 0.1 \mathrm{M} \mathrm{NaCl} \) \( 0.1 \mathrm{M} \) sucrose \( 0.1 \mathrm{M} \mathrm{HCl} \)
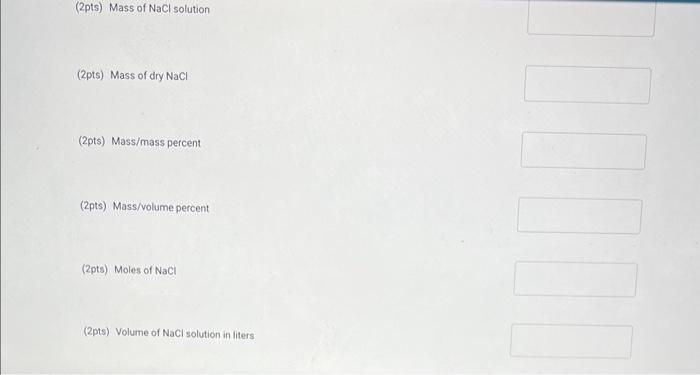
Write an equation for the dissolution of \( \mathrm{HCl}_{4} \mathrm{NH}_{4} \mathrm{OH}_{4} \) and \( \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH} \) in water. (14pts) Concentration Complete the following entries to determine the concentration of the NaCl solution. Be sure to report answers to the correct number of significant figures. Mass of empty evaporating dish: \( 29.114 \mathrm{~g} \) Volume of \( \mathrm{NaCl} \) solution. \( 10.10 \mathrm{~mL} \) Mass of dish and \( \mathrm{NaCl} \) solution: \( 41864 \mathrm{~g} \) Mass of dish and dry \( \mathrm{NaCl} \) : \( 31.864 q \)
(2pts) Mass of \( \mathrm{NaCl} \) solution (2pts) Mass of dry \( \mathrm{NaCl} \) (2pts) Mass/mass percent (2pts) Mass/volume percent (2pts) Moles of \( \mathrm{NaCl} \) (2pts) Volume of \( \mathrm{NaCl} \) solution in liters
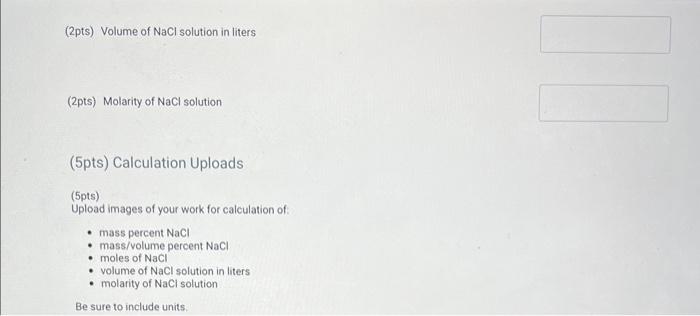
(2pts) Volume of \( \mathrm{NaCl} \) solution in liters (2pts) Molarity of \( \mathrm{NaCl} \) solution (5pts) Calculation Uploads (5pts) Upload images of your work for calculation of: - mass percent \( \mathrm{NaCl} \) - mass/volume percent \( \mathrm{NaCl} \) - moles of \( \mathrm{NaCl} \) - volume of \( \mathrm{NaCl} \) solution in liters - molarity of \( \mathrm{NaCl} \) solution Be sure to include units.
Expert Answer
Mass of NaCl solution = (41.864 - 29.114) g = 12.75 g Mass of dry NaCl = (31.864 -29.11