Home /
Expert Answers /
Chemical Engineering /
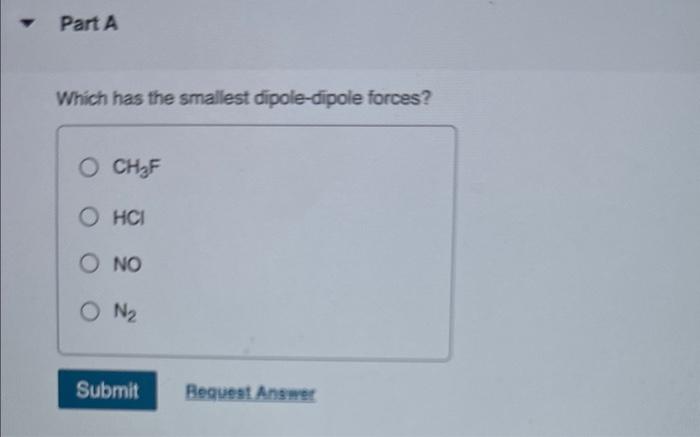
which-has-the-smallest-dipole-dipole-forces-ch3f-hcl-no-n2-pa992
Expert Answer
N2 is a non polar molecule. Triple bond is shared between two nitrogen atoms. The dipole mo