Home /
Expert Answers /
Chemistry /
when-measuring-the-mass-of-your-flask-you-should-weigh-to-the-nearest-a-0-01-mathrm-g-b-pa538
(Solved): When measuring the mass of your flask, you should weigh to the nearest a. \( 0.01 \mathrm{~g} \) b. ...
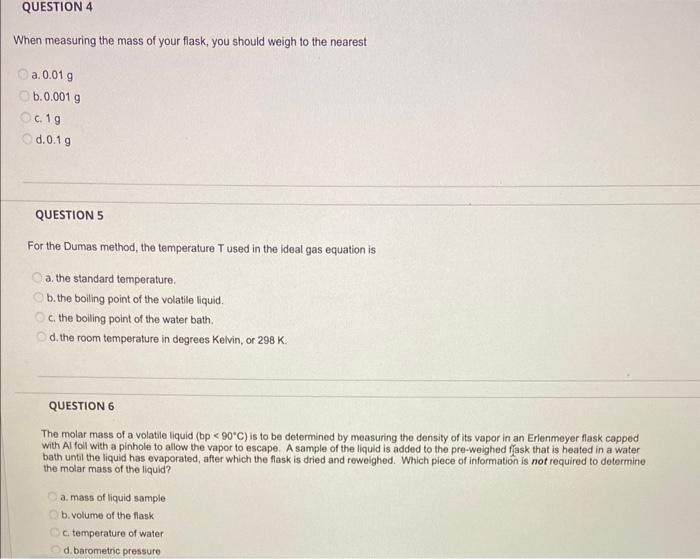
When measuring the mass of your flask, you should weigh to the nearest a. \( 0.01 \mathrm{~g} \) b. \( 0.001 \mathrm{~g} \) c. \( 1 \mathrm{~g} \) d. \( 0.1 \mathrm{~g} \) QUESTION 5 For the Dumas method, the temperature T used in the ideal gas equation is a. the standard temperature. b. the boiling point of the volatile liquid. c. the bolling point of the water bath. d. the room temperature in degrees Kelvin, or \( 298 \mathrm{~K} \). QUESTION 6 The molar mass of a volatile liquid (bp \( <90^{\circ} \mathrm{C} \) ) is to be detormined by moasuring the density of its vapor in an Erienmeyer flask cappod bath until the liquid has evaporated, affer which the flask is dried and rowoighed. Which plece of information is not required to dotormine the molar mass of the liquid? a. mass of liquid sample b. volume of the flask c. temperature of water d. barometric pressure