Home /
Expert Answers /
Chemical Engineering /
when-a-chemical-substance-is-analyzed-using-absorption-spectroscopy-the-spectrometer-generates-a-s-pa397
(Solved): When a chemical substance is analyzed using absorption spectroscopy, the spectrometer generates a s ...
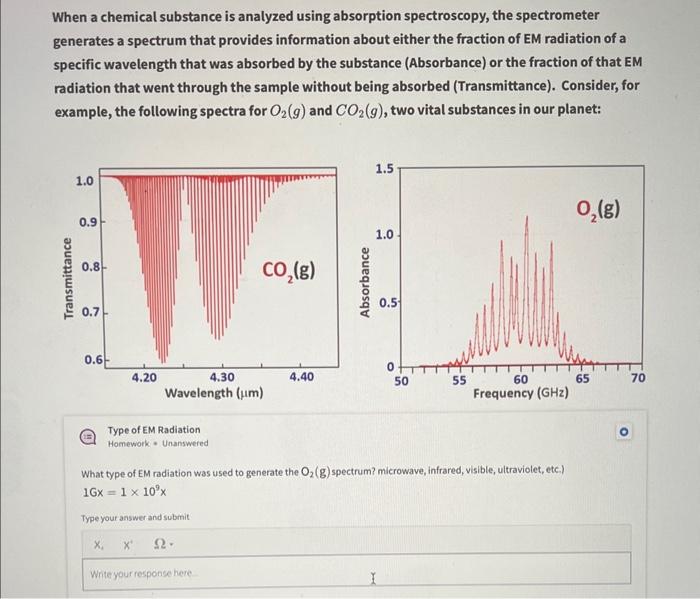
When a chemical substance is analyzed using absorption spectroscopy, the spectrometer generates a spectrum that provides information about either the fraction of EM radiation of a specific wavelength that was absorbed by the substance (Absorbance) or the fraction of that EM radiation that went through the sample without being absorbed (Transmittance). Consider, for example, the following spectra for \( \mathrm{O}_{2}(g) \) and \( \mathrm{CO}_{2}(g) \), two vital substances in our planet: Type of EM Radiation Homework - Unanswered What type of EM radiation was used to generate the \( \mathrm{O}_{2}(\mathrm{~g}) \) spectrum? microwave, infrared, visible, ultraviolet, etc.) \( 16 x=1 \times 10^{9} x \) Type your answer and submit \[ x_{4} \quad x^{4} \quad \Omega \text {. } \] Write your response here
Expert Answer
The frequency of light used is in high energy range of GHz. This high energy range corresponds to electro