Home /
Expert Answers /
Chemistry /
what-volume-in-gas-would-be-produced-by-the-complete-reaction-of-2-93-g-of-al-solid-at-stp-accor-pa343
(Solved): What volume in gas would be produced by the complete reaction of 2.93 g of Al solid at STP accor ...
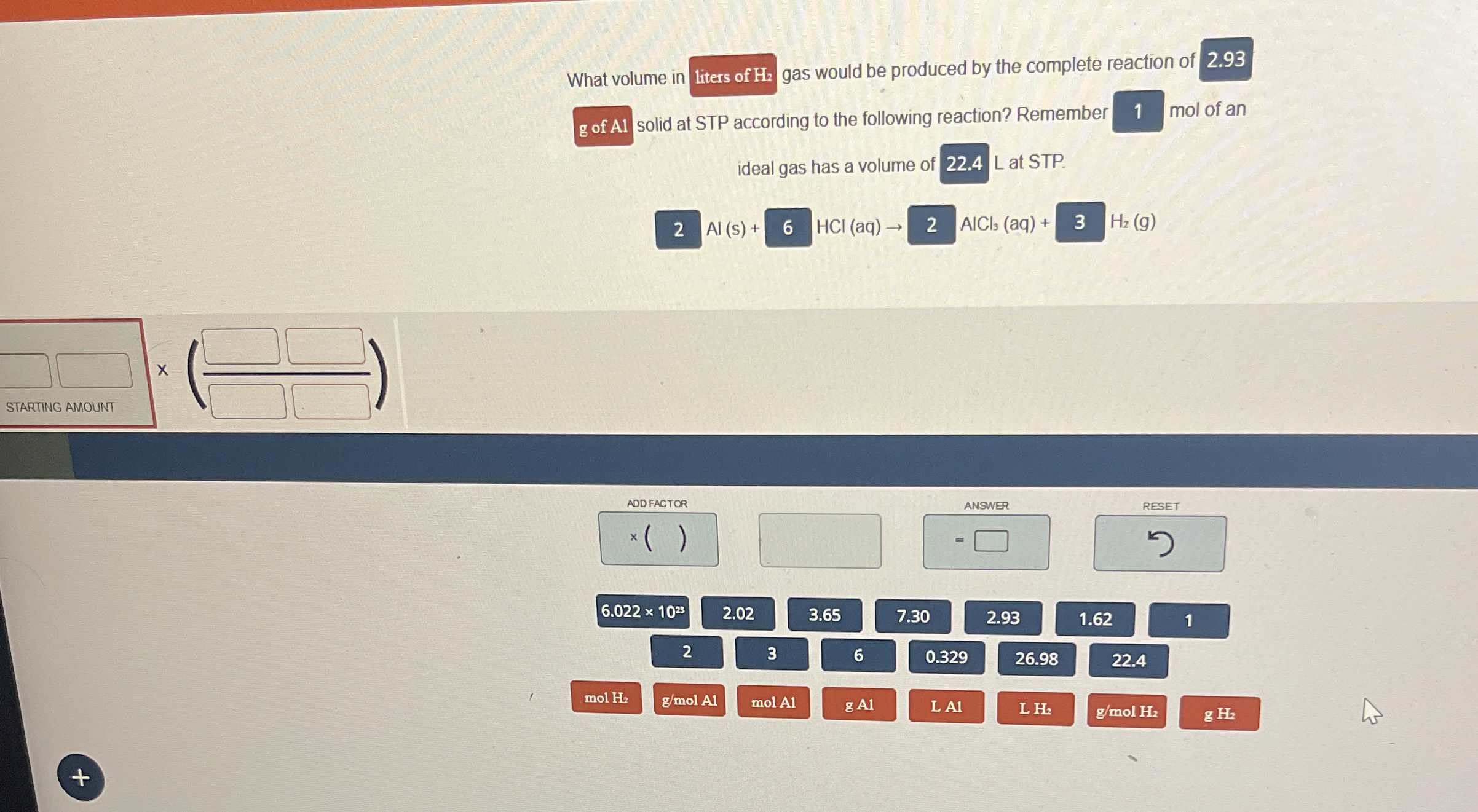
What volume in
◻gas would be produced by the complete reaction of 2.93 g of Al solid at STP according to the following reaction? Remember
q,mol of an ideal gas has a volume of
q,L at STP.
Al(s)+,HCl(aq)->,|AlCl_(3)(aq)+,|H_(2)(g)
◻
Al(s)+
HCl(aq)->
AlCl_(3)(aq)+
H_(2)(g)
◻
x
◻
◻STARTING AMOUNT
(/bar (◻))
◻