Home /
Expert Answers /
Chemistry /
what-is-the-total-energy-for-one-mole-of-photons-that-possesses-a-wavelength-of-430-mathrm-nm-pa879
(Solved): What is the total energy for one mole of photons that possesses a wavelength of \( 430 \mathrm{~nm} ...
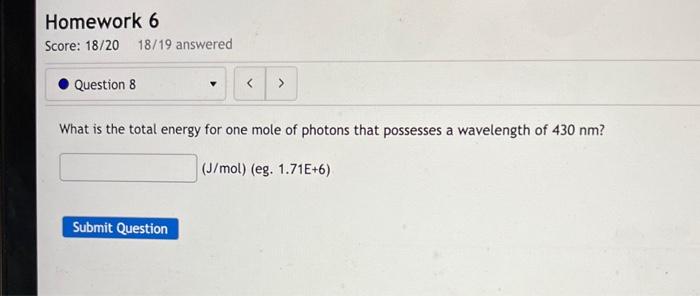

What is the total energy for one mole of photons that possesses a wavelength of \( 430 \mathrm{~nm} \) ? \[ (\mathrm{J} / \mathrm{mol}) \text { (eg. } 1.71 \mathrm{E}+6) \]
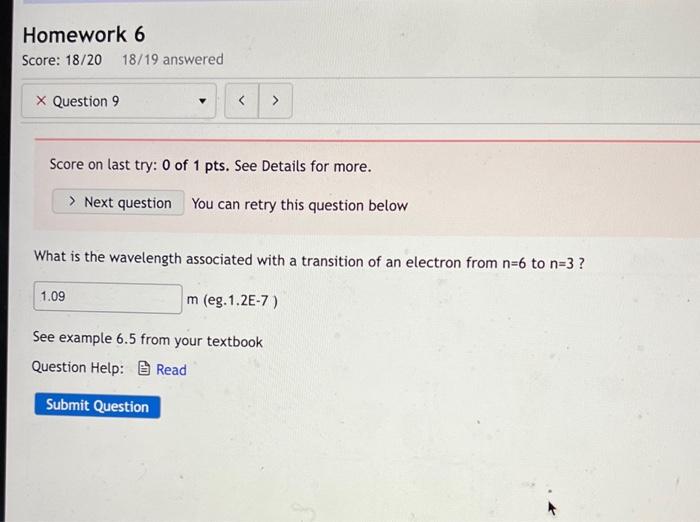
Score on last try: 0 of 1 pts. See Details for more. You can retry this question below What is the wavelength associated with a transition of an electron from \( n=6 \) to \( n=3 \) ? m (eg.1.2E-7) See example \( 6.5 \) from your textbook Question Help: G Read
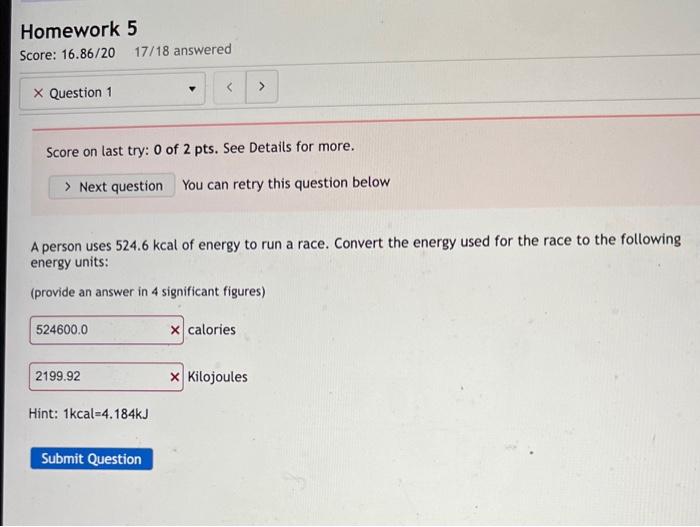
Score on last try: 0 of 2 pts. See Details for more. You can retry this question below A person uses \( 524.6 \mathrm{kcal} \) of energy to run a race. Convert the energy used for the race to the following energy units: (provide an answer in 4 significant figures) calories Kilojoules Hint: \( 1 \mathrm{kcal}=4.184 \mathrm{~kJ} \)
\[ \mathrm{CH}_{4}+2 \mathrm{O}_{2} \rightarrow \mathrm{CO}_{2}+2 \mathrm{H}_{2} \mathrm{O} \quad \Delta \mathrm{H}=890 \mathrm{~kJ} / \mathrm{mol} \] How much heat is released when \( 6.8 \mathrm{~g} \) of methane gas is burned in a constant pressure system?