Home /
Expert Answers /
Chemistry /
what-is-the-net-cell-reaction-for-the-iron-silver-voltaic-cell-express-your-answer-as-a-chemical-e-pa914
(Solved): What is the net cell reaction for the iron-silver voltaic cell? Express your answer as a chemical e ...
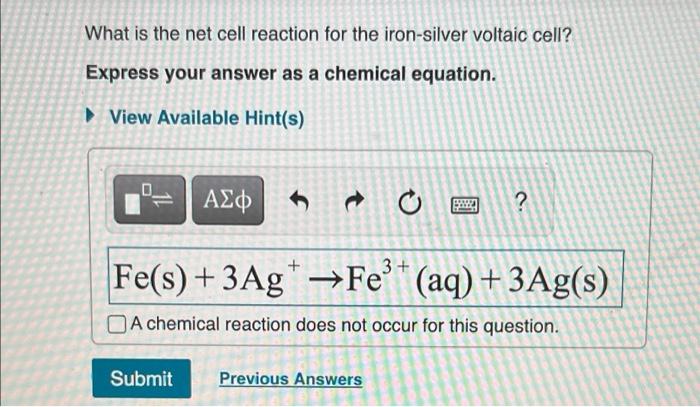

What is the net cell reaction for the iron-silver voltaic cell? Express your answer as a chemical equation. View Available Hint(s) ??? 6 t o S. 2 + 3+ Fe(s) +3Ag* ?Fe®* (aq) + 3Ag(s) A chemical reaction does not occur for this question. Submit Previous Answers
Construction of a voltaic cell Consider a iron-silver voltaic cell that is constructed such that one half-cell consists of the iron, Fe, electrode immersed in a Fe(NO3)3 solution, and the other half-cell consists of the silver, Ag, electrode immersed in a AgNO3 solution. The two electrodes are connected by a copper wire. The Fe electrode acts as the anode, and the Ag electrode acts as the cathode. To maintain electric neutrality, you add a KNO3 salt bridge separating the two half-cells. Use this information to solve Parts B, C, and D.