Home /
Expert Answers /
Chemistry /
we-can-draw-three-inequivalent-lewis-structures-for-the-bromate-ion-mathrm-bro-3-pa706
(Solved): We can draw three inequivalent Lewis structures for the bromate ion, \( \mathrm{BrO}_{3}{ }^{-} . ...
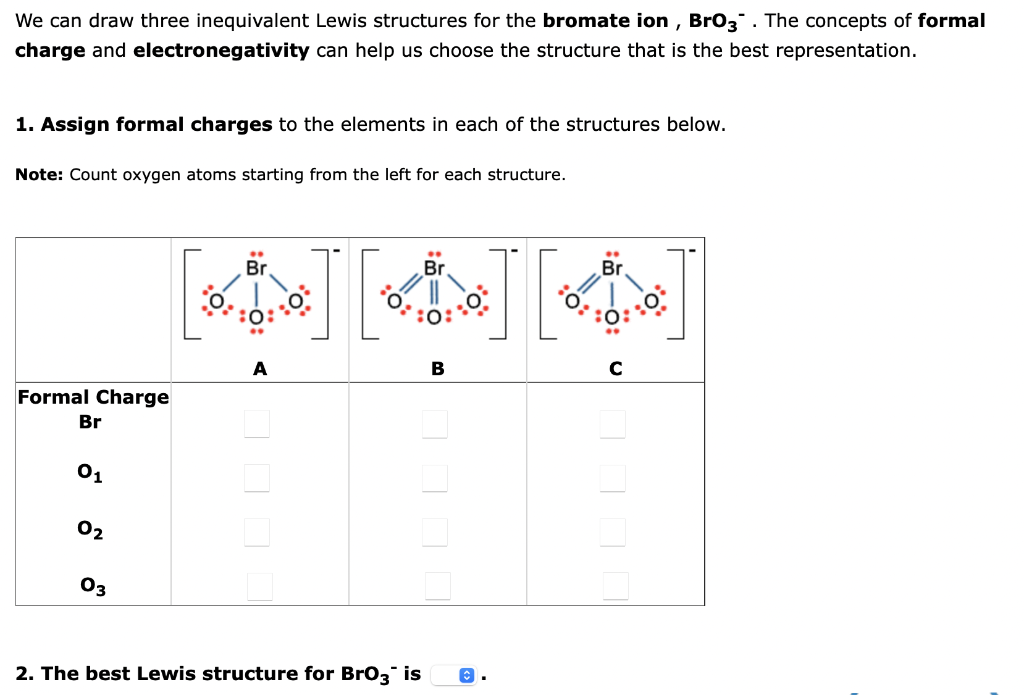
We can draw three inequivalent Lewis structures for the bromate ion, \( \mathrm{BrO}_{3}{ }^{-} . \)The concepts of formal charge and electronegativity can help us choose the structure that is the best representation. 1. Assign formal charges to the elements in each of the structures below. Note: Count oxygen atoms starting from the left for each structure. 2. The best Lewis structure for \( \mathrm{BrO}_{3}{ }^{-} \)is
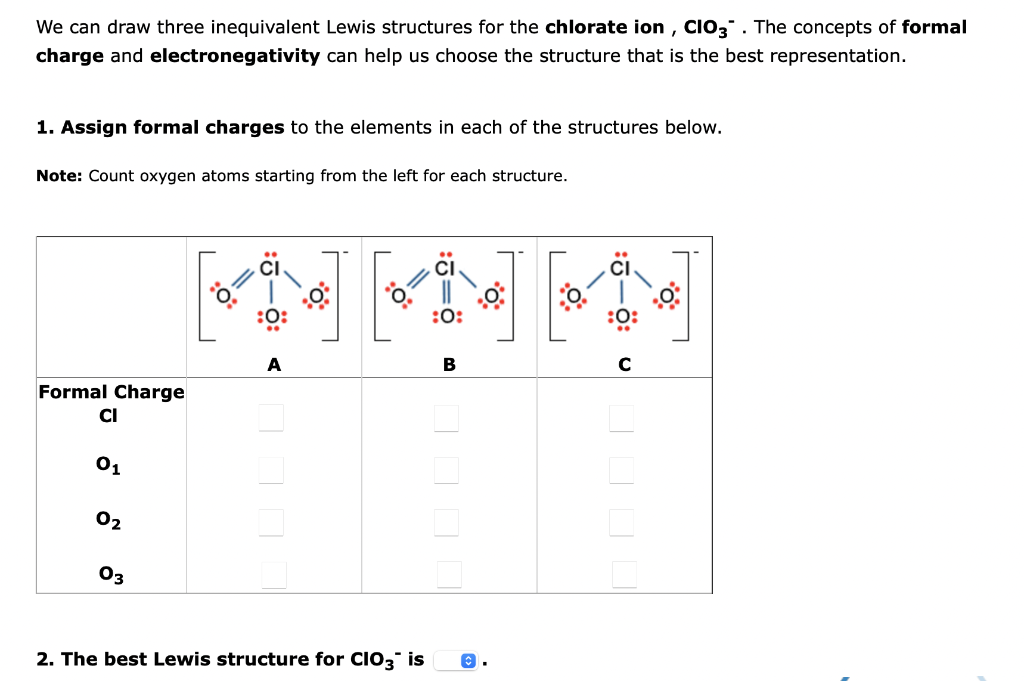
We can draw three inequivalent Lewis structures for the chlorate ion, \( \mathbf{C l O}_{3}{ }^{-} \). The concepts of formal charge and electronegativity can help us choose the structure that is the best representation. 1. Assign formal charges to the elements in each of the structures below. Note: Count oxygen atoms starting from the left for each structure. 2. The best Lewis structure for \( \mathrm{ClO}_{3}{ }^{-} \)is