Home /
Expert Answers /
Chemistry /
vsepr-theory-predict-the-molecular-shape-of-tef-5-using-vsepr-theory-1-draw-for-yourself-the-pa573
(Solved): VSEPR theory Predict the molecular shape of TeF 5 - using VSEPR theory. (1) Draw for yourself the ...
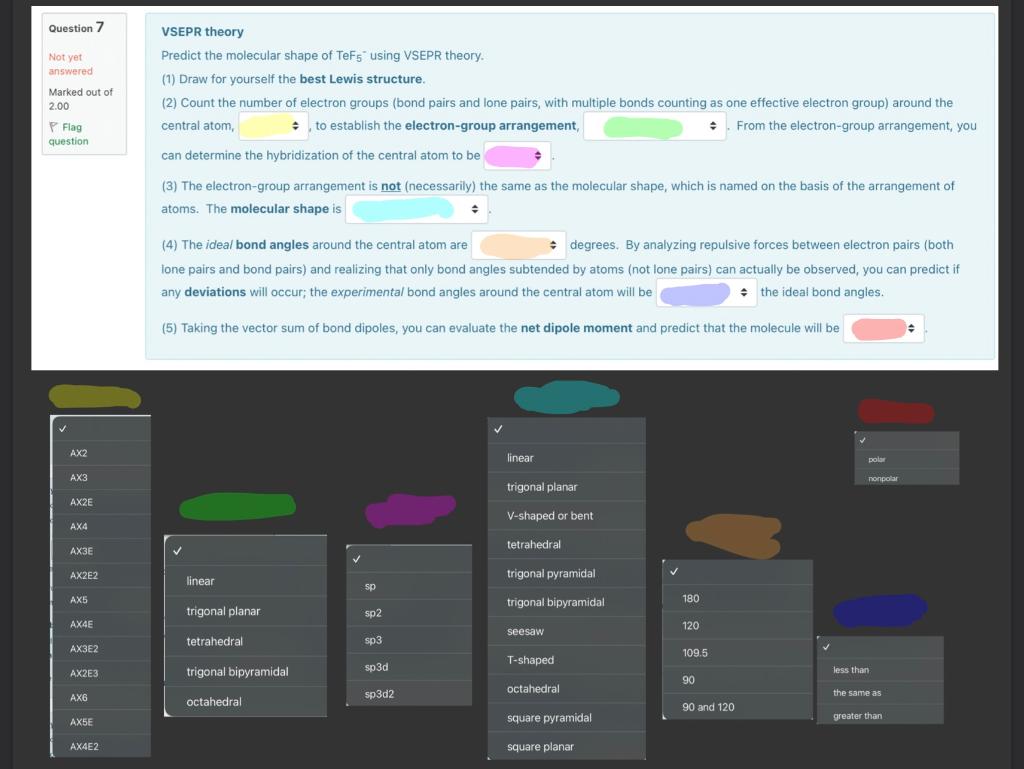
VSEPR theory Predict the molecular shape of TeF 5 - using VSEPR theory. (1) Draw for yourself the best Lewis structure. (2) Count the number of electron groups (bond pairs and lone pairs, with multiple bonds counting as one effective electron group) around the central atom, to establish the electron-group arrangement, From the electron-group arrangement, you can determine the hybridization of the central atom to be (3) The electron-group arrangement is not (necessarily) the same as the molecular shape, which is named on the basis of the arrangement of atoms. The molecular shape is (4) The ideal bond angles around the central atom are degrees. By analyzing repulsive forces between electron pairs (both lone pairs and bond pairs) and realizing that only bond angles subtended by atoms (not lone pairs) can actually be observed, you can predict if any deviations will occur; the experimental bond angles around the central atom will be the ideal bond angles. (5) Taking the vector sum of bond dipoles, you can evaluate the net dipole moment and predict that the molecule will be