Home /
Expert Answers /
Chemistry /
van-der-waals-constants-for-hypothetical-gases-mind-the-units-you-are-familiar-with-the-ideal-g-pa788
(Solved): van der Waals constants for hypothetical gases (mind the units!) You are familiar with the ideal g ...
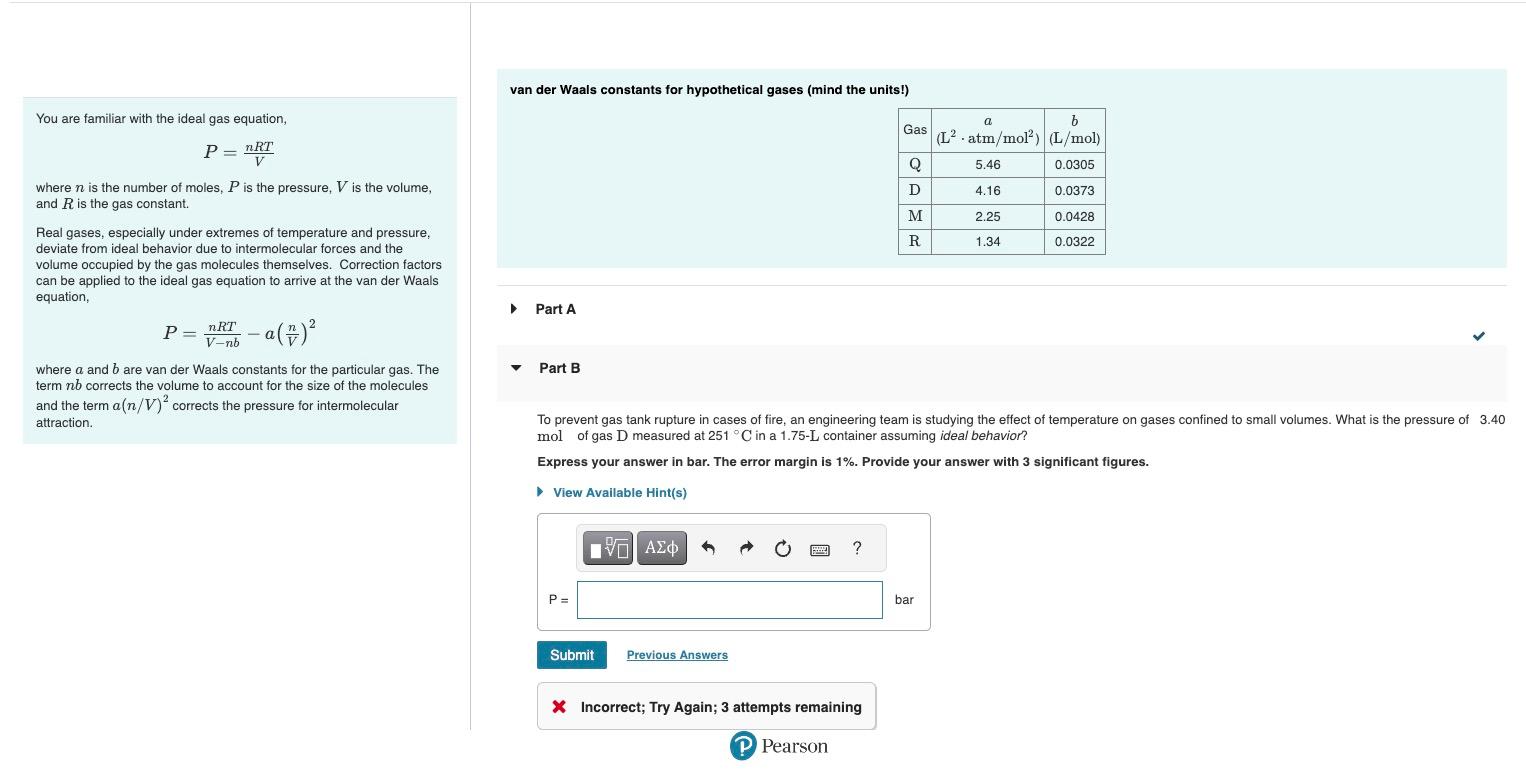
van der Waals constants for hypothetical gases (mind the units!) You are familiar with the ideal gas equation, \[ P=\frac{n R T}{V} \] where \( n \) is the number of moles, \( P \) is the pressure, \( V \) is the volume, and \( R \) is the gas constant. Real gases, especially under extremes of temperature and pressure, deviate from ideal behavior due to intermolecular forces and the volume occupied by the gas molecules themselves. Correction factors can be applied to the ideal gas equation to arrive at the van der Waals equation, \[ P=\frac{n R T}{V-n b}-a\left(\frac{n}{V}\right)^{2} \quad \text { • Part A } \] where \( a \) and \( b \) are van der Waals constants for the particular gas. The Part B term \( n b \) corrects the volume to account for the size of the molecules and the term \( a(n / V)^{2} \) corrects the pressure for intermolecular attraction. To prevent gas tank rupture in cases of fire, an engineering team is studying the effect of temperature on gases confined to small volumes. What is the pressure of \( 3.40 \) \( \mathrm{mol} \) of gas \( \mathrm{D} \) measured at \( 251^{\circ} \mathrm{C} \) in a \( 1.75-\mathrm{L} \) container assuming ideal behavior? Express your answer in bar. The error margin is \( 1 \% \). Provide your answer with 3 significant figures. Incorrect; Try Again; 3 attempts remaining
Expert Answer
Ideal gas follows the equation of PV=nRT