Home /
Expert Answers /
Chemistry /
v-determine-the-strongest-attractive-force-intermolecular-force-imf-in-the-following-molecules-l-pa167
(Solved): v Determine the strongest attractive force (intermolecular force; IMF) in the following molecules. L ...
v
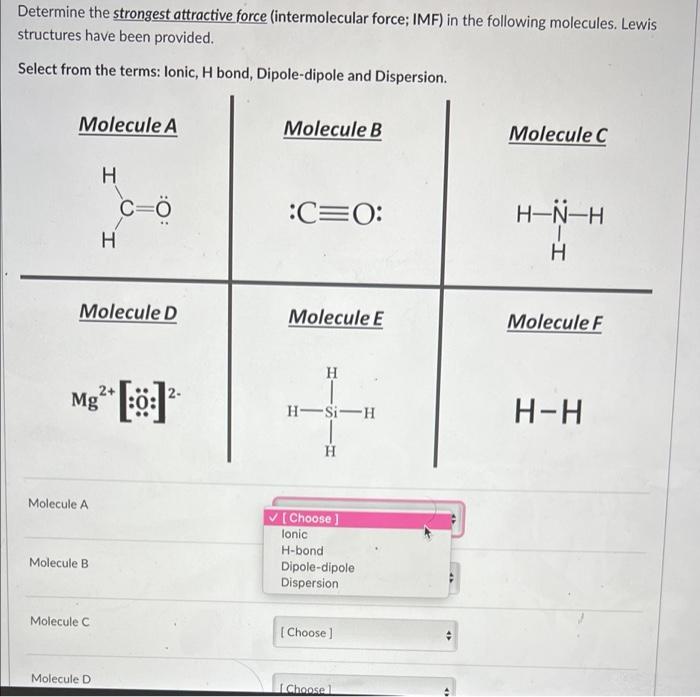

Determine the strongest attractive force (intermolecular force; IMF) in the following molecules. Lewis structures have been provided. Select from the terms: lonic, H bond, Dipole-dipole and Dispersion. Molecule A Mg Molecule A Molecule D Molecule B Molecule C H Molecule Di H 2+ C=Ö :0: Molecule B :C=O: Molecule E H H-Si-H H ?[Choose ] lonic H-bond Dipole-dipole Dispersion [Choose] [Choose 1 Molecule C H-N-H H Molecule F H-H
Expert Answer
Molecule A contains dipole-dipole interactions, molecule B contains dipole - dipole int