Home /
Expert Answers /
Chemistry /
using-the-titration-curve-below-determine-the-following-5-points-a-is-the-analyte-a-strong-acid-pa831
(Solved): Using the titration curve below, determine the following: (5 points) a) Is the analyte a strong acid ...
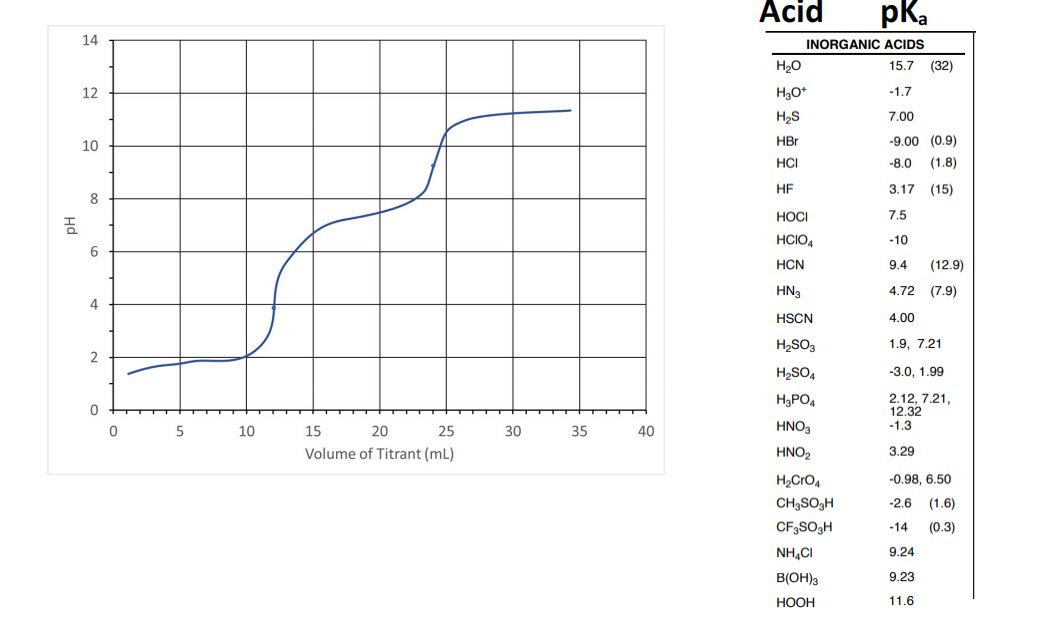
Using the titration curve below, determine the following: (5 points)
a) Is the analyte a strong acid, weak acid, or polyprotic acid? What is the titrant (what kind of
substance)? What do the dots on the titration curve represent?
b) What is the ½ equivalency point of the titration? If more than one ½ equivalency point exists,
report the volume of titrant and pH of each.
c) If the titration was performed with 0.30 M NaOH, what is the concentration of the acid solution?
d) Using the table of pKa values for inorganic acids below: can you determine which species was
the analyte? (Ignore pKa values in parentheses on the table.)
5 14 12 10 8 6 4 2 0 0 5 10 15 20 25 Volume of Titrant (ml) 30 35 40 Acid H?O H?O+ H?S HBr HCI HF HOCI HCIO4 HCN HN3 HSCN H?SO3 H?SO4 H?PO4 HNO3 HNO? H?CrO4 CH3SO3H CF3SO3H NH?C B(OH)3 HOOH pka INORGANIC ACIDS 15.7 (32) -1.7 7.00 -9.00 (0.9) -8.0 (1.8) 3.17 (15) 7.5 -10 9.4 4.72 (7.9) 4.00 1.9, 7.21 -3.0, 1.99 2.12, 7.21, 12.32 -1.3 3.29 -0.98, 6.50 -2.6 (1.6) -14 (0.3) 9.24 9.23 11.6 (12.9)
Expert Answer
A) it is for polyprotic acid titration curve titrant is 0.1 M naoh solution