Home /
Expert Answers /
Chemistry /
using-the-rules-learned-in-class-draw-the-lewis-structure-of-mathrm-sio-2-and-use-it-to-pa513
(Solved): Using the rules learned in class, draw the Lewis structure of \( \mathrm{SiO}_{2} \) and use it to ...
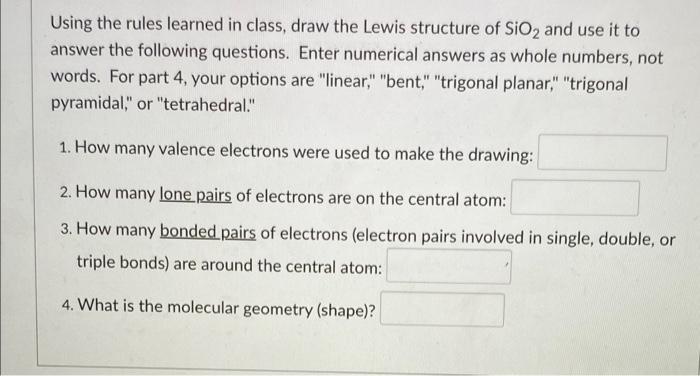
Using the rules learned in class, draw the Lewis structure of \( \mathrm{SiO}_{2} \) and use it to answer the following questions. Enter numerical answers as whole numbers, not words. For part 4, your options are "linear," "bent," "trigonal planar," "trigonal pyramidal," or "tetrahedral." 1. How many valence electrons were used to make the drawing: 2. How many lone pairs of electrons are on the central atom: 3. How many bonded pairs of electrons (electron pairs involved in single, double, or triple bonds) are around the central atom: 4. What is the molecular geometry (shape)?