Home /
Expert Answers /
Chemistry /
using-hess-39-s-law-nbsp-using-hess-39-law-to-determine-the-delta-mathrm-h-mathrm-f-of-pa227
(Solved): Using Hess's Law Using Hess' Law to Determine the \( \Delta \mathrm{H}_{\mathrm{f}} \) of \( \ ...
Using Hess's Law
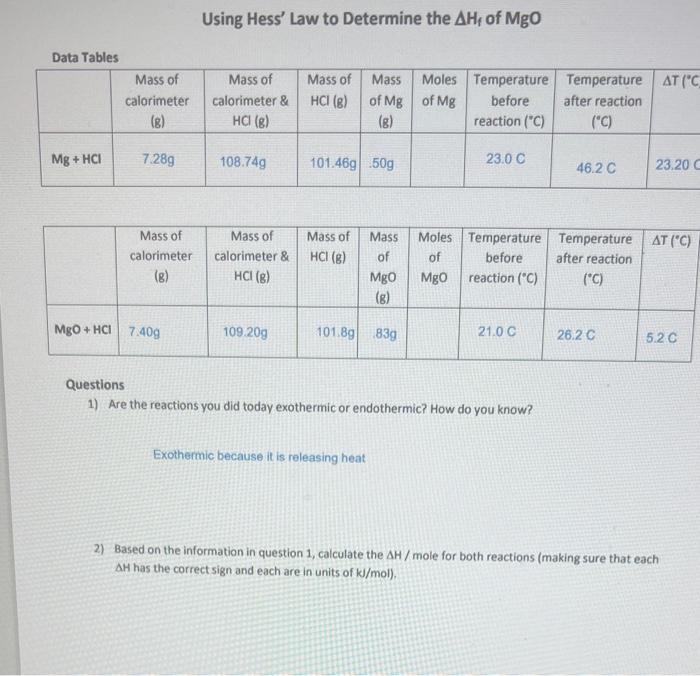

Using Hess' Law to Determine the \( \Delta \mathrm{H}_{\mathrm{f}} \) of \( \mathrm{MgO} \) Questions 1) Are the reactions you did today exothermic or endothermic? How do you know? Exothermic because it is releasing heat 2) Based on the information in question 1 , calculate the \( \Delta H \) / mole for both reactions (making sure that each AH has the correct sign and each are in units of \( \mathrm{kJ} / \mathrm{mol} \) ).
Expert Answer
According to Hess's law, the overall change in enthalpy of a chemical reaction is constant regardless of the direction the reaction proceeds. Molar mass of Mg = 24.30 g/mol Molar mass of MgO = 40.30 g/mol 1 Celsius heat unit to kilojoule = 1.8991 kil