Home /
Expert Answers /
Chemistry /
using-electronegativity-values-from-the-table-below-classify-the-bond-formed-between-each-pair-of-pa963
(Solved): Using electronegativity values from the table below, classify the bond formed between each pair of ...
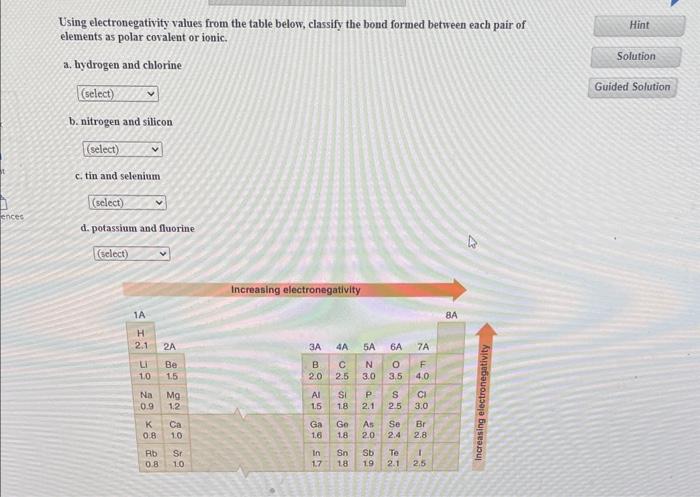
Using electronegativity values from the table below, classify the bond formed between each pair of elements as polar covalent or ionic. a. hydrogen and chlorine b. nitrogen and silicon c. tin and selenium d. potassium and fluorine
Expert Answer
please note :- i ) if the electronegativity difference between two atoms atoms is 1.9,