Home /
Expert Answers /
Chemical Engineering /
two-consecutive-reactions-occur-according-to-the-reaction-scheme-shown-below-ak1ik2-pa188
(Solved): Two consecutive reactions occur according to the reaction scheme shown below: Ak1Ik2 ...
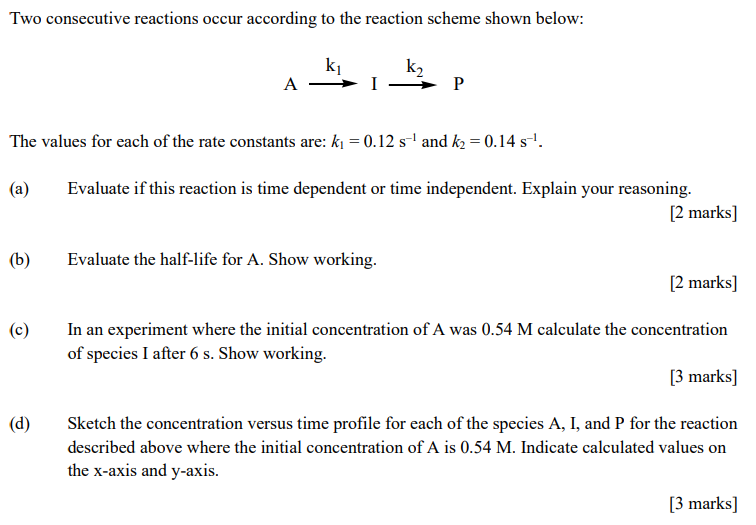
Two consecutive reactions occur according to the reaction scheme shown below: The values for each of the rate constants are: and . (a) Evaluate if this reaction is time dependent or time independent. Explain your reasoning. marks (b) Evaluate the half-life for A. Show working. marks (c) In an experiment where the initial concentration of A was calculate the concentration of species I after . Show working. [3 marks (d) Sketch the concentration versus time profile for each of the species A, I, and P for the reaction described above where the initial concentration of is . Indicate calculated values on the -axis and -axis. [3 marks]
Expert Answer
To determine if the reaction is time dependent or time independent, we need to examine the rate constants and their relative magnitudes.(a) If the rate constant for the first reaction, k?, is significantly larger than the rate constant for the second...