Home /
Expert Answers /
Chemistry /
tlc-analysis-based-on-the-tlc-data-shown-below-comment-on-the-relative-polarity-of-reactants-vs-pr-pa342
(Solved): TLC analysis: based on the TLC data shown below comment on the relative polarity of reactants vs. pr ...
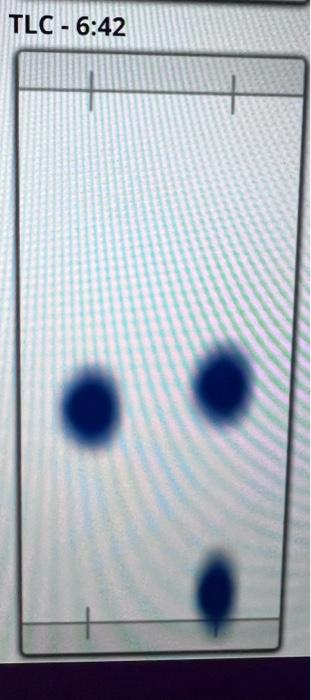
TLC analysis: based on the TLC data shown below comment on the relative polarity of reactants vs. product, the completion of the reaction, and calculate approximate Rf values.
 beginning of reaction
beginning of reaction
 15 min after
15 min after
 end of reaction
end of reaction
reactants: butyraldehyde, water, potassium hydroxide
product: 2-ethyl-hex-2-enal
 beginning of reaction
beginning of reaction 15 min after
15 min after end of reaction
end of reaction
TLC - 6:42
TLC - \( 6: 48 \)
Expert Answer
Reactant is less polar than product because the product forms a spot at the lower end of the TLC that