Home /
Expert Answers /
Chemistry /
this-experiment-had-be-add-1-0-gram-of-calcium-chloride-dihydrate-to-25-mls-of-distilled-water-in-a-pa925
(Solved): This experiment had be add 1.0 gram of Calcium chloride dihydrate to 25 mls of distilled water in a ...
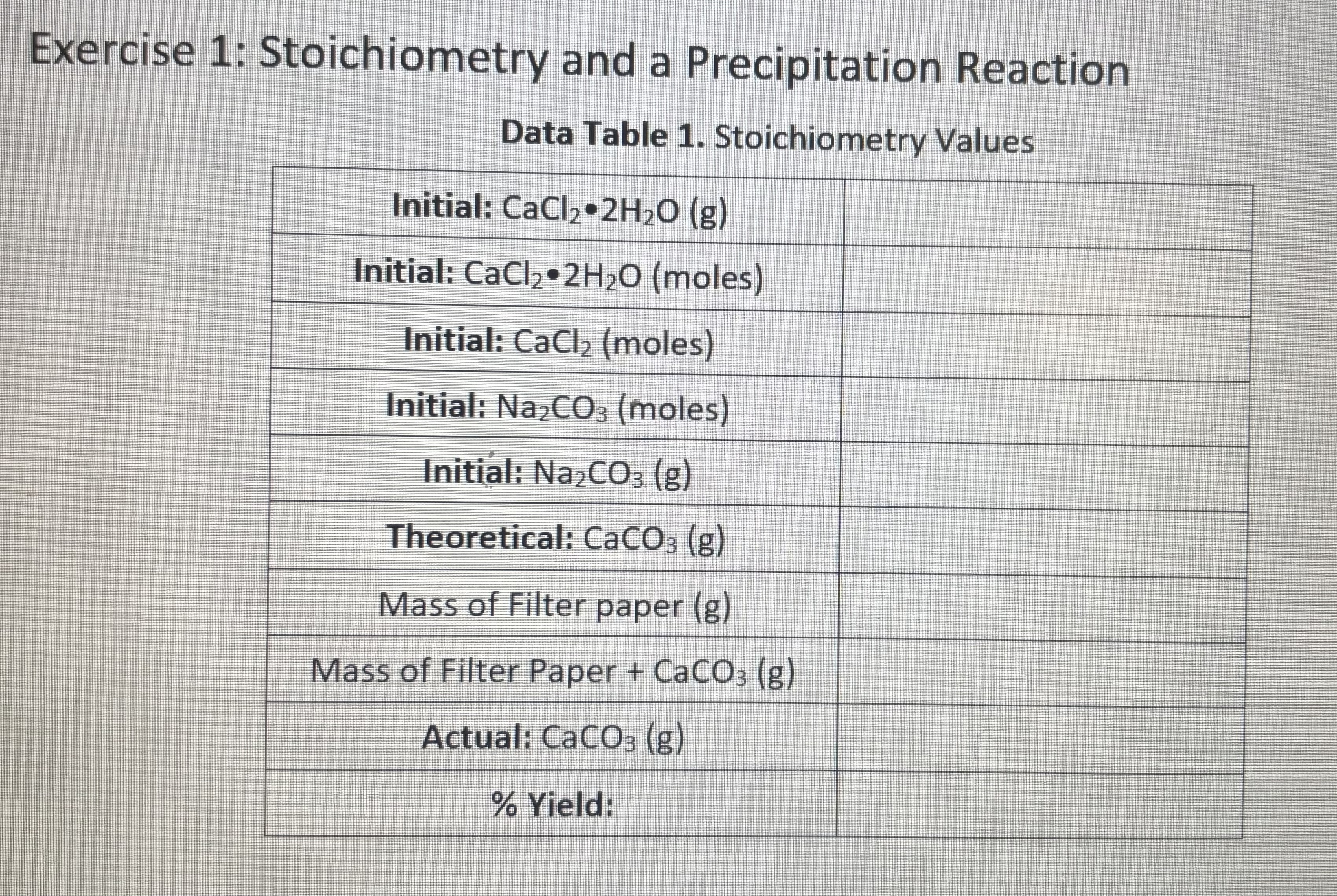
This experiment had be add 1.0 gram of Calcium chloride dihydrate to 25 mls of distilled water in a 100 ml beaker. The before weight of the beaker and water was 73.3 i added 1.0 grams of calcium chloride dihydrate and the weight afterwards was 74.7 grams. How do I use this information to solve the CaCl2problems? Also, how do find out how many moles and then grams of sodium carbonate are needed to reach stoichiometric quantities?