Home /
Expert Answers /
Chemistry /
there-are-four-possible-structural-isomers-of-mathrm-c-8-mathrm-h-10-that-contain-a-b-pa171
(Solved): There are four possible structural isomers of \( \mathrm{C}_{8} \mathrm{H}_{10} \) that contain a b ...
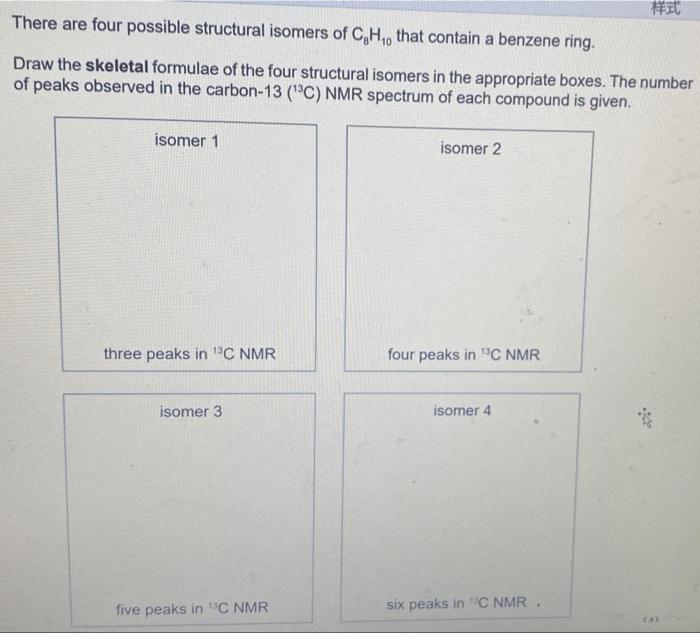
There are four possible structural isomers of \( \mathrm{C}_{8} \mathrm{H}_{10} \) that contain a benzene ring. Draw the skeletal formulae of the four structural isomers in the appropriate boxes. The number of peaks observed in the carbon-13 \( \left({ }^{13} \mathrm{C}\right) \mathrm{NMR} \) spectrum of each compound is aiven.
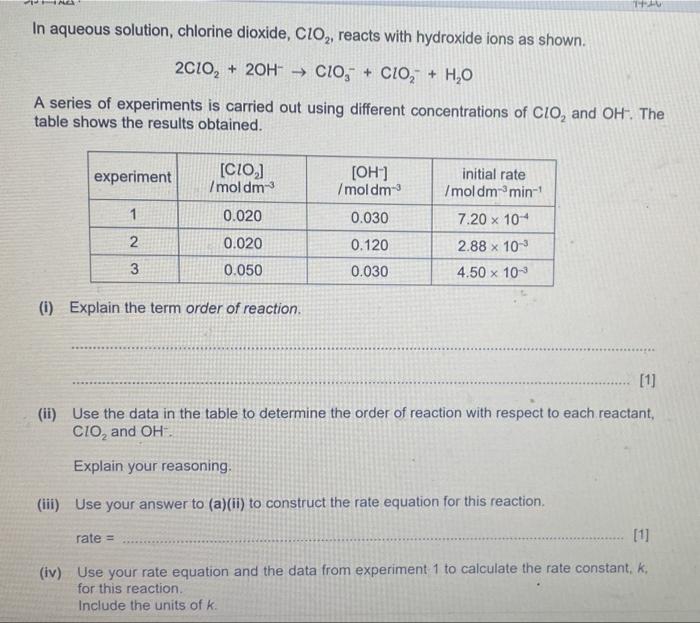
In aqueous solution, chlorine dioxide, \( \mathrm{ClO}_{2} \), reacts with hydroxide ions as shown. \[ 2 \mathrm{ClO}_{2}+2 \mathrm{OH}^{-} \rightarrow \mathrm{ClO}_{3}^{-}+\mathrm{ClO}_{2}^{-}+\mathrm{H}_{2} \mathrm{O} \] A series of experiments is carried out using different concentrations of \( \mathrm{ClO}_{2} \) and \( \mathrm{OH}^{-} \). The table shows the results obtained. (i) Explain the term order of reaction. (ii) Use the data in the table to determine the order of reaction with respect to each reactant, \( \mathrm{ClO}_{2} \) and \( \mathrm{OH}^{-} \) Explain your reasoning. (iii) Use your answer to (a)(ii) to construct the rate equation for this reaction. rate \( = \) (iv) Use your rate equation and the data from experiment 1 to calculate the rate constant, \( k \), for this reaction. Include the units of \( k \).
Expert Answer
2.i. Order of a reaction is nothing but the power to which co