Home /
Expert Answers /
Chemistry /
the-standard-enthalpy-of-combustion-reaction-of-1-00mol-of-acetone-c3h6o-is-1790kj-pa613
(Solved): The standard enthalpy of combustion reaction of 1.00mol of acetone (C3H6O) is 1790kJ. ...
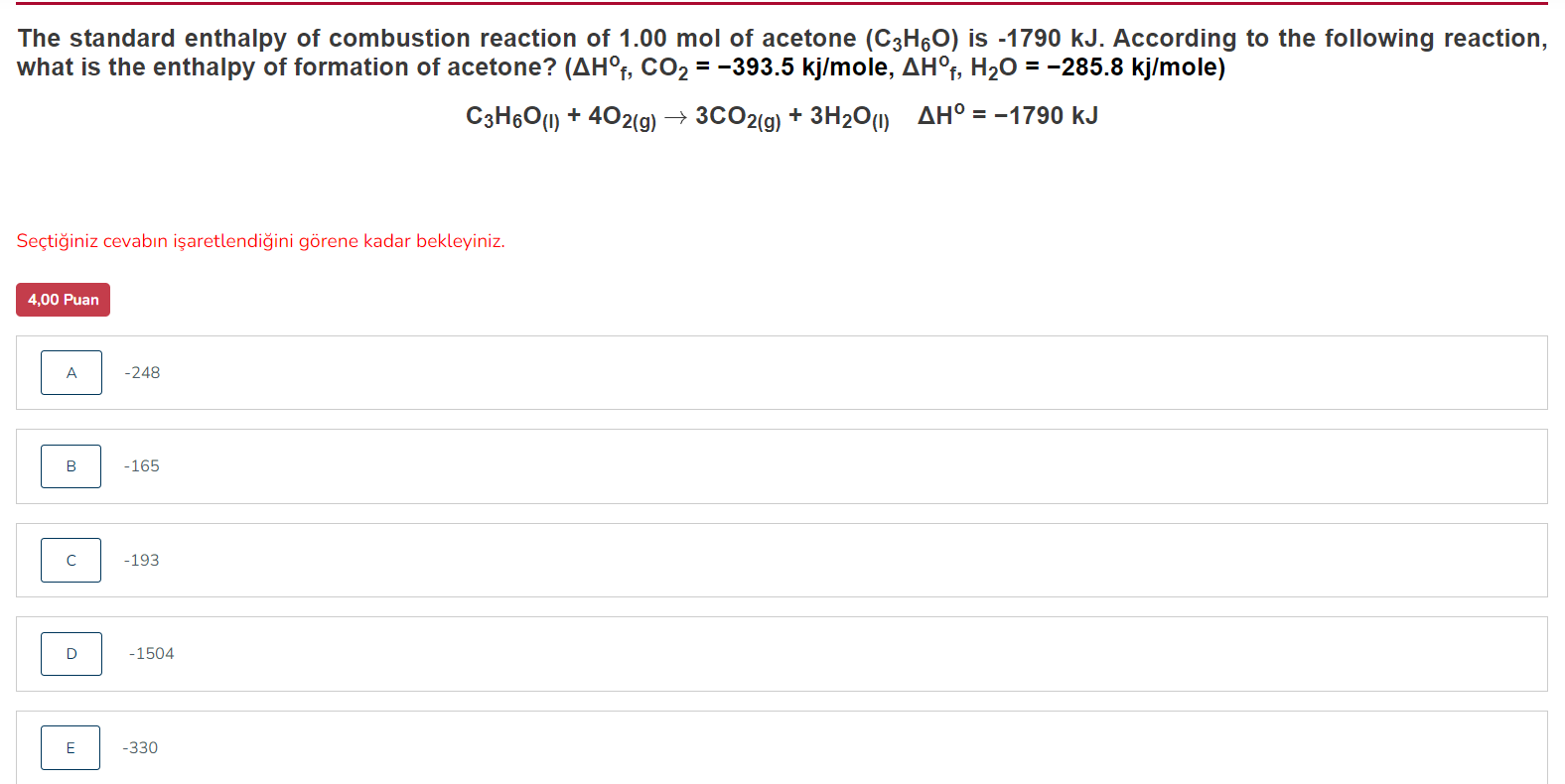
The standard enthalpy of combustion reaction of of acetone is . According to the following reaction, what is the enthalpy of formation of acetone? Seçti?iniz cevab?n i?aretlendi?ini görene kadar bekleyiniz.
Expert Answer
The given chemical reaction in the question along with enthalpy