Home /
Expert Answers /
Chemical Engineering /
the-specific-reaction-rate-constant-k-is-a-strong-function-of-temperature-according-to-the-f-pa326
(Solved): The specific reaction rate constant \( k \) is a strong function of temperature according to the f ...
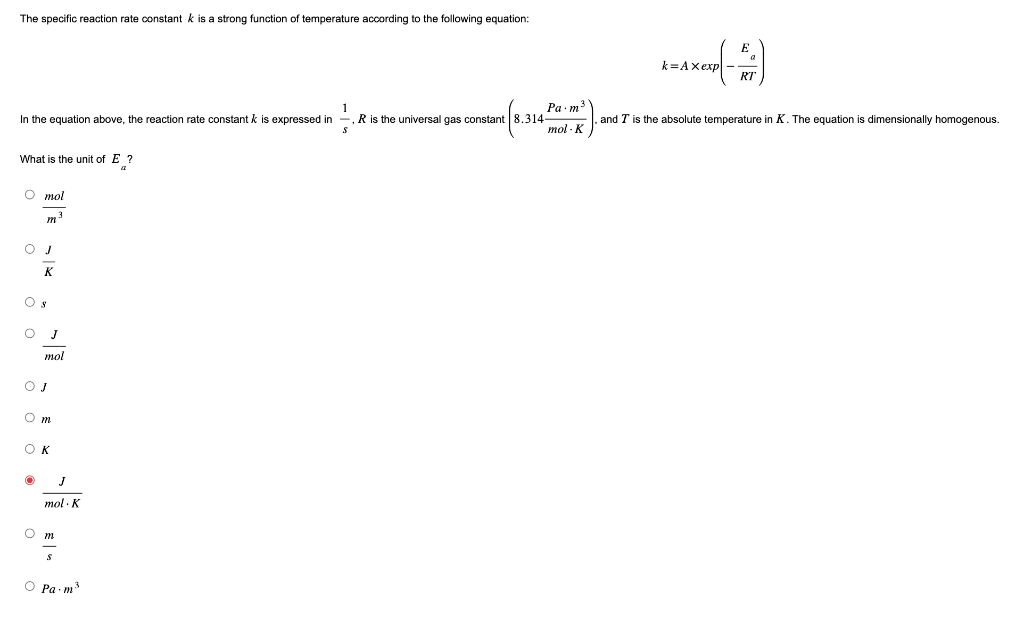
The specific reaction rate constant \( k \) is a strong function of temperature according to the following equation: \( \quad E\left(-\frac{a}{R T}\right) \) In the equation above, the reaction rate constant \( k \) is expressed in \( \frac{1}{s}, R \) is the universal gas constant \( \left(8.314 \frac{P a \cdot m^{3}}{m o l \cdot K}\right) \), and \( T \) is the absolute temperature in \( K \). The equation is dimensionally homogenous. What is the unit of \( E_{a} \) ? \( \frac{m o l}{m^{3}} \) \( \frac{J}{K} \) \( s \) \( \frac{J}{m o l} \) \( J \) \( m \) \( K \) \[ \frac{J}{m o l \cdot K} \] \( \frac{m}{s} \) \[ P a \cdot m^{3} \]