(Solved): The reaction of \( \mathrm{N}_{2} \mathrm{O}_{5}(\mathrm{~g}) \) and \( \math ...
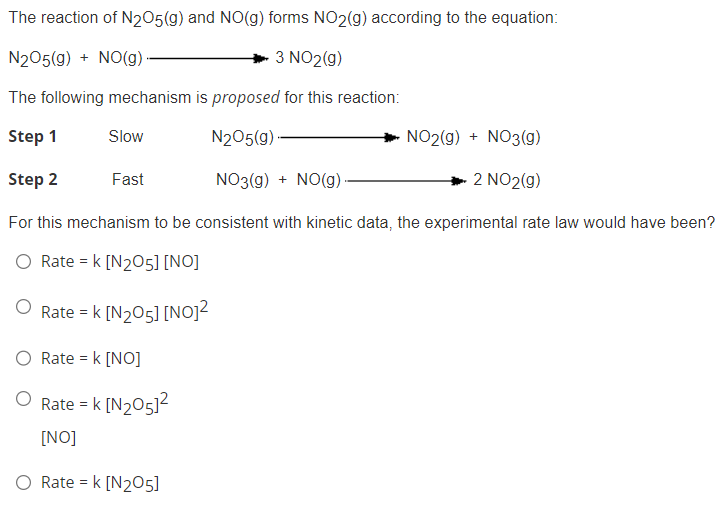
The reaction of \( \mathrm{N}_{2} \mathrm{O}_{5}(\mathrm{~g}) \) and \( \mathrm{NO}(\mathrm{g}) \) forms \( \mathrm{NO}_{2}(\mathrm{~g}) \) according to the equation: \[ \mathrm{N}_{2} \mathrm{O}_{5}(\mathrm{~g})+\mathrm{NO}(\mathrm{g}) \longrightarrow 3 \mathrm{NO}_{2}(\mathrm{~g}) \] The following mechanism is proposed for this reaction: Step 1 Slow \( \mathrm{N}_{2} \mathrm{O}_{5}(\mathrm{~g}) \rightarrow \mathrm{NO}_{2}(\mathrm{~g})+\mathrm{NO}_{3}(\mathrm{~g}) \) Step 2 Fast \( \quad \mathrm{NO}_{3}(\mathrm{~g})+\mathrm{NO}(\mathrm{g}) \longrightarrow 2 \mathrm{NO}_{2}(\mathrm{~g}) \) For this mechanism to be consistent with kinetic data, the experimental rate law would have been? \[ \begin{array}{l} \text { Rate }=k\left[\mathrm{~N}_{2} \mathrm{O}_{5}\right][\mathrm{NO}] \\ \text { Rate }=k\left[\mathrm{~N}_{2} \mathrm{O}_{5}\right]\left[\mathrm{NO}^{2}\right. \\ \text { Rate }=k\left[\mathrm{NO}^{2}\right. \\ \text { Rate }=k\left[\mathrm{~N}_{2} \mathrm{O}_{5}\right]^{2} \\ {[\mathrm{NO}]} \\ \text { Rate }=k\left[\mathrm{~N}_{2} \mathrm{O}_{5}\right] \end{array} \]