Home /
Expert Answers /
Chemistry /
the-reaction-of-ethane-gas-left-mathrm-c-2-mathrm-h-6-right-with-chlorine-gas-prod-pa927
(Solved): The reaction of ethane gas \( \left(\mathrm{C}_{2} \mathrm{H}_{6}\right) \) with chlorine gas prod ...
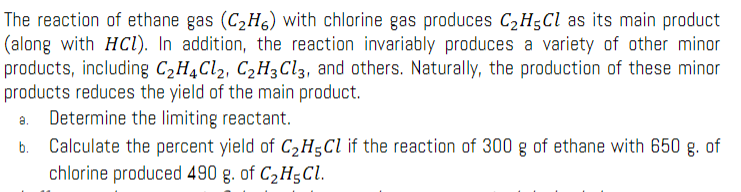
The reaction of ethane gas \( \left(\mathrm{C}_{2} \mathrm{H}_{6}\right) \) with chlorine gas produces \( \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{Cl} \) as its main product (along with \( \mathrm{HCl} \) ). In addition, the reaction invariably produces a variety of other minor products, including \( \mathrm{C}_{2} \mathrm{H}_{4} \mathrm{Cl}_{2}, \mathrm{C}_{2} \mathrm{H}_{3} \mathrm{Cl}_{3} \), and others. Naturally, the production of these minor products reduces the yield of the main product. a. Determine the limiting reactant. b. Calculate the percent yield of \( \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{Cl} \) if the reaction of \( 300 \mathrm{~g} \) of ethane with \( 650 \mathrm{~g} \). of chlorine produced \( 490 \mathrm{~g} \). of \( \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{Cl} \).