Home /
Expert Answers /
Chemical Engineering /
the-oxidation-of-ethylene-to-produce-ethylene-oxide-proceeds-according-to-the-equation-2-mathrm-pa433
(Solved): The oxidation of ethylene to produce ethylene oxide proceeds according to the equation \[ 2 \mathrm ...
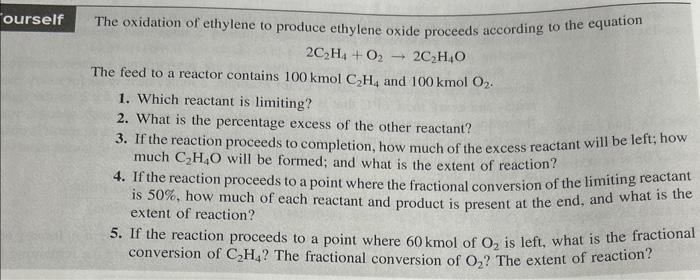
The oxidation of ethylene to produce ethylene oxide proceeds according to the equation \[ 2 \mathrm{C}_{2} \mathrm{H}_{4}+\mathrm{O}_{2} \rightarrow 2 \mathrm{C}_{2} \mathrm{H}_{4} \mathrm{O} \] The feed to a reactor contains \( 100 \mathrm{kmol} \mathrm{C}_{2} \mathrm{H}_{4} \) and \( 100 \mathrm{kmol} \mathrm{O} 2_{2} \). 1. Which reactant is limiting? 2. What is the percentage excess of the other reactant? 3. If the reaction proceeds to completion, how much of the excess reactant will be left; how much \( \mathrm{C}_{2} \mathrm{H}_{4} \mathrm{O} \) will be formed; and what is the extent of reaction? 4. If the reaction proceeds to a point where the fractional conversion of the limiting reactant is \( 50 \% \), how much of each reactant and product is present at the end, and what is the extent of reaction? 5. If the reaction proceeds to a point where \( 60 \mathrm{kmol} \) of \( \mathrm{O}_{2} \) is left, what is the fractional conversion of \( \mathrm{C}_{2} \mathrm{H}_{4} \) ? The fractional conversion of \( \mathrm{O}_{2} \) ? The extent of reaction?