Home /
Expert Answers /
Chemistry /
the-nernst-equation-is-one-of-the-most-important-equations-in-quad-part-a-electrochemistry-pa953
(Solved): The Nernst equation is one of the most important equations in \( \quad \) Part A electrochemistry. ...
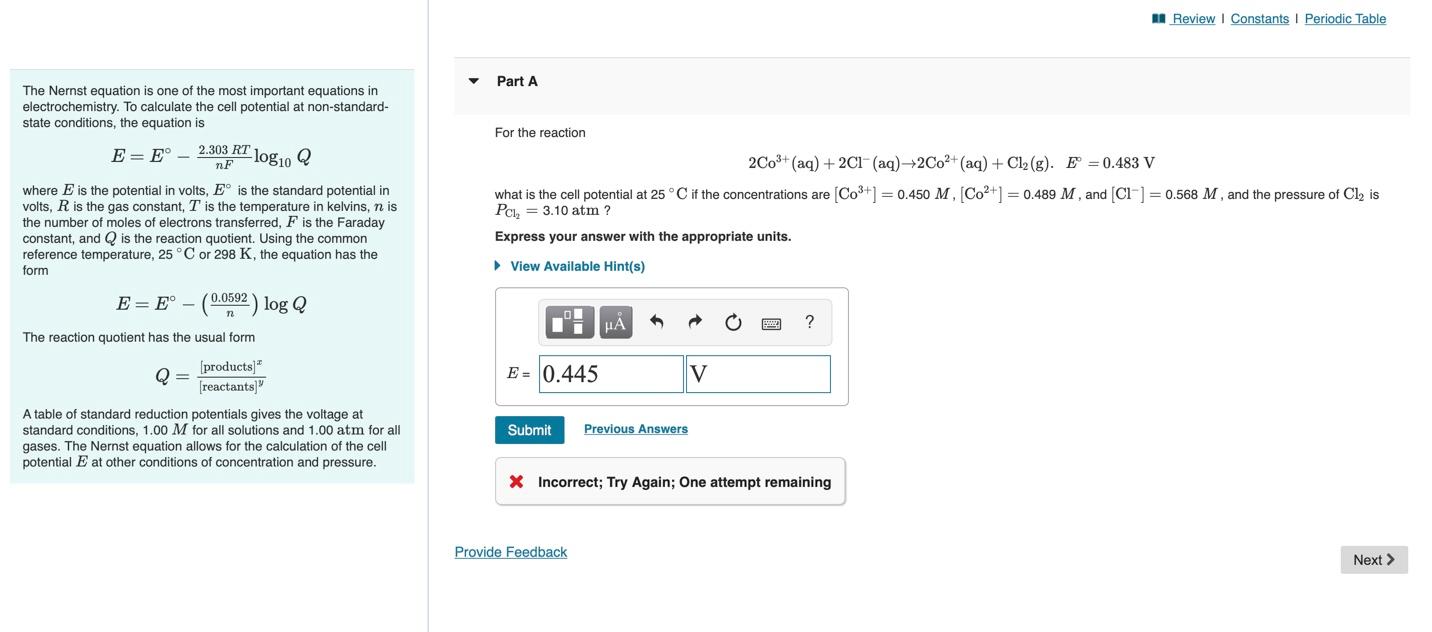
The Nernst equation is one of the most important equations in \( \quad \) Part A electrochemistry. To calculate the cell potential at non-standardstate conditions, the equation is \[ E=E^{\circ}-\frac{2.303 R T}{n F} \log _{10} Q \] For the reaction \[ 2 \mathrm{Co}^{3+}(\mathrm{aq})+2 \mathrm{Cl}^{-}(\mathrm{aq}) \rightarrow 2 \mathrm{Co}^{2+}(\mathrm{aq})+\mathrm{Cl}_{2}(\mathrm{~g}) . \quad E^{\circ}=0.483 \mathrm{~V} \] where \( E \) is the potential in volts, \( E^{\circ} \) is the standard potential in volts, \( R \) is the gas constant, \( T \) is the temperature in kelvins, \( n \) is what is the cell potential at \( 25^{\circ} \mathrm{C} \) if the concentrations are \( \left[\mathrm{Co}^{3+}\right]=0.450 M,\left[\mathrm{Co}^{2+}\right]=0.489 M \), and \( \left[\mathrm{Cl}^{-}\right]=0.568 \mathrm{M}^{\text {, }} \) and the pressure of \( \mathrm{Cl}_{2} \) is the number of moles of electrons transferred, \( F \) is the Faraday \( P_{\mathrm{Cl}_{2}}=3.10 \mathrm{~atm} ? \) constant, and \( Q \) is the reaction quotient. Using the common Express your answer with the appropriate units. reference temperature, \( 25^{\circ} \mathrm{C} \) or \( 298 \mathrm{~K} \), the equation has the form \[ E=E^{\circ}-\left(\frac{0.0592}{n}\right) \log Q \] The reaction quotient has the usual form \[ Q=\frac{[\text { products }]^{x}}{[\text { reactants }]^{y}} \] A table of standard reduction potentials gives the voltage at standard conditions, \( 1.00 \mathrm{M} \) for all solutions and \( 1.00 \mathrm{~atm} \) for all gases. The Nernst equation allows for the calculation of the cell potential \( E \) at other conditions of concentration and pressure. 4 Incorrect; Try Again; One attempt remaining
Expert Answer
Given, the cell reaction is 2Co3+ (aq) + 2C1-(aq.) 2Co2+ (aq.) + Cl2 (g), E°cell = 0.483V Temperature = T =