Home /
Expert Answers /
Chemistry /
the-mineral-muscovite-consists-of-silicate-sheets-ionically-bonded-with-potassium-k-l-cations-and-pa748
(Solved): The mineral muscovite consists of silicate sheets ionically bonded with potassium (K+l) cations and ...
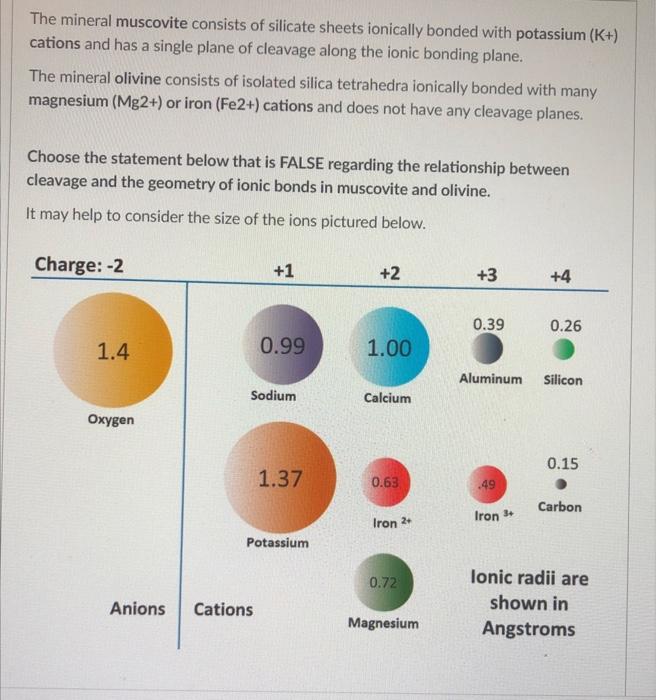
The mineral muscovite consists of silicate sheets ionically bonded with potassium cations and has a single plane of cleavage along the ionic bonding plane. The mineral olivine consists of isolated silica tetrahedra ionically bonded with many magnesium or iron cations and does not have any cleavage planes. Choose the statement below that is FALSE regarding the relationship between cleavage and the geometry of ionic bonds in muscovite and olivine. It may help to consider the size of the ions pictured below.
Can't see the image? Click the following link to view a high-resolution PDF version: Image PDF Link . The potassium ionic bonds in muscovite are all in one plane, making it very weak, whereas in olivine the bonds are on all sides such that there is no plane that is weaker than another. Some of the silica tetrahedra in olivine bond to each other, strengthening the olivine mineral through covalent bonds. The large potassium ion in muscovite means that the ionic bond distance in muscovite is longer and weaker than the ionic bonds in olivine.
Expert Answer
Some of the silica tetrahedra in olivine bond to each other, strengthening the olivine miner