Home /
Expert Answers /
Chemistry /
the-mass-spectrum-of-an-organic-compound-shows-the-relative-abundances-of-mathrm-m-to-be-pa430
(Solved): The mass spectrum of an organic compound shows the relative abundances of \( \mathrm{M} \) to be \ ...
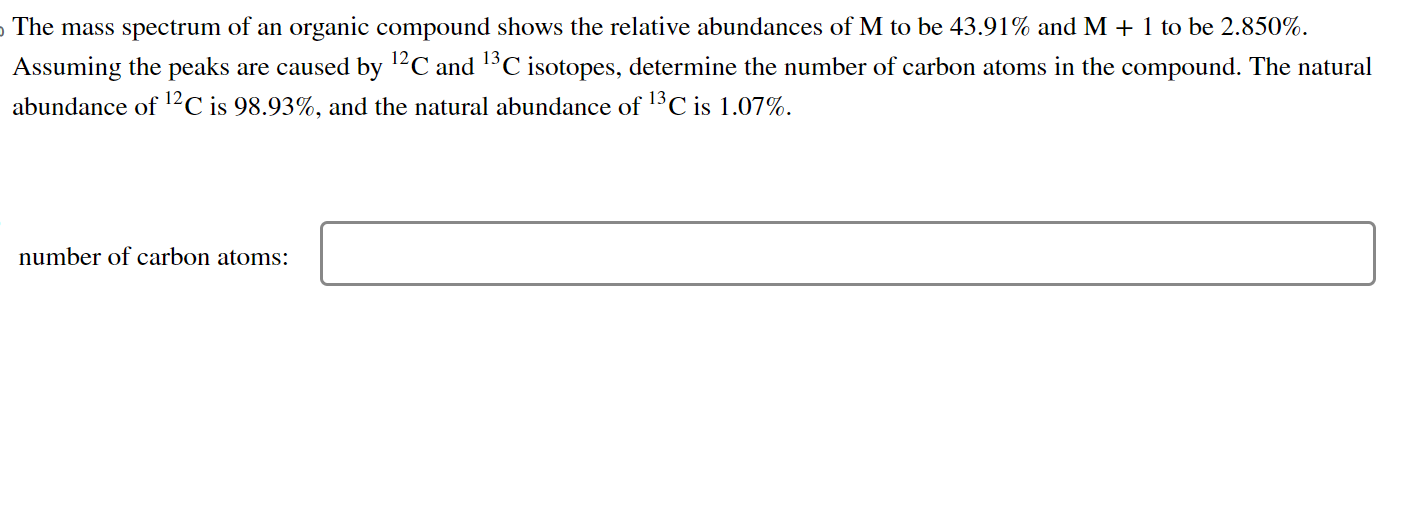
The mass spectrum of an organic compound shows the relative abundances of \( \mathrm{M} \) to be \( 43.91 \% \) and \( \mathrm{M}+1 \) to be \( 2.850 \% \). Assuming the peaks are caused by \( { }^{12} \mathrm{C} \) and \( { }^{13} \mathrm{C} \) isotopes, determine the number of carbon atoms in the compound. The natural abundance of \( { }^{12} \mathrm{C} \) is \( 98.93 \% \), and the natural abundance of \( { }^{13} \mathrm{C} \) is \( 1.07 \% \).
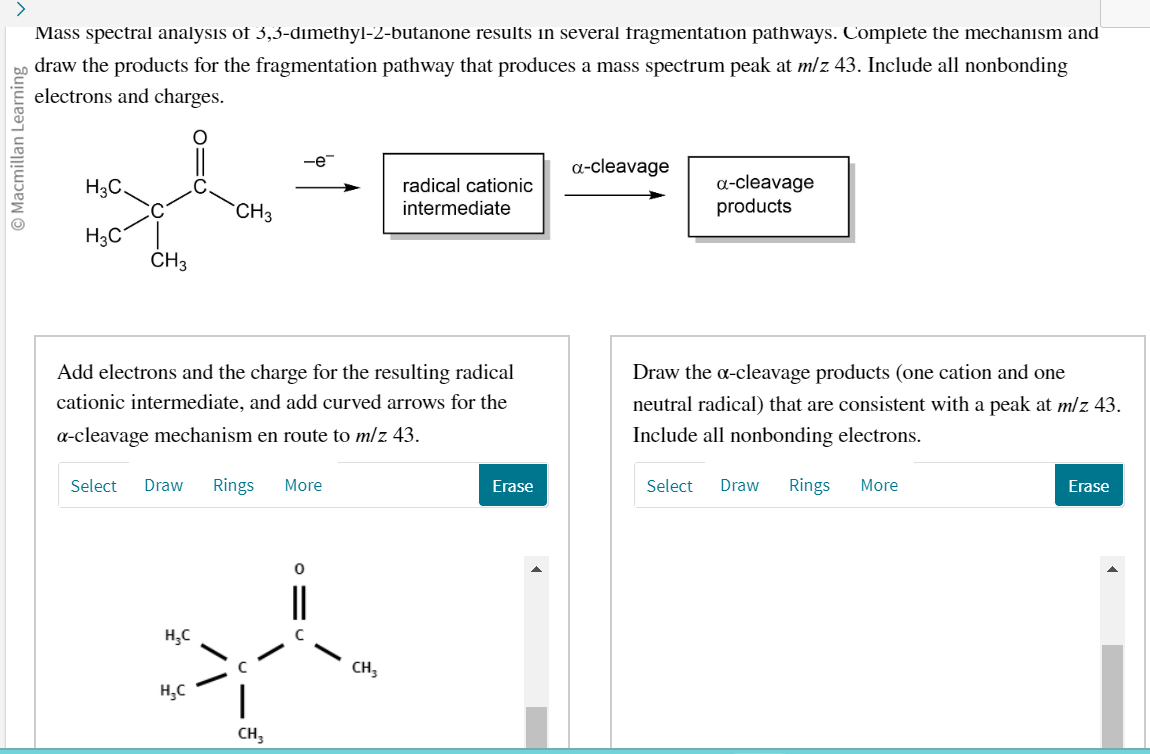
Mass spectral analysis of 3,3-d?methyl-2-butanone results in several tragmentation pathways. Complete the mechanism and draw the products for the fragmentation pathway that produces a mass spectrum peak at \( \mathrm{m} / \mathrm{z} 43 \). Include all nonbonding electrons and charges. Add electrons and the charge for the resulting radical Draw the \( \alpha \)-cleavage products (one cation and one cationic intermediate, and add curved arrows for the neutral radical) that are consistent with a peak at \( \mathrm{m} / \mathrm{z} 43 \). \( \alpha \)-cleavage mechanism en route to \( \mathrm{m} / \mathrm{z} 43 \). Include all nonbonding electrons.
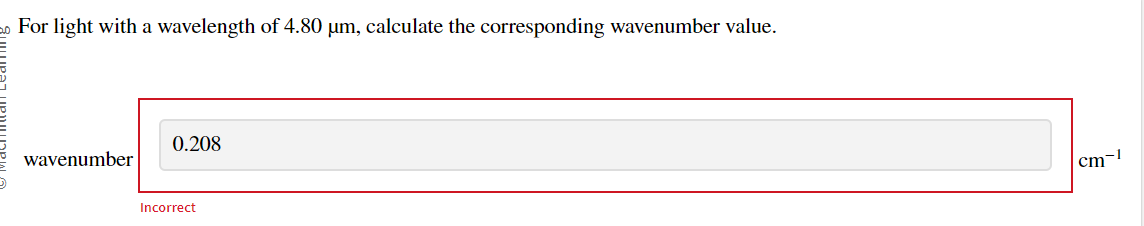
For light with a wavelength of \( 4.80 \mu \mathrm{m} \), calculate the corresponding wavenumber value. wavenumber
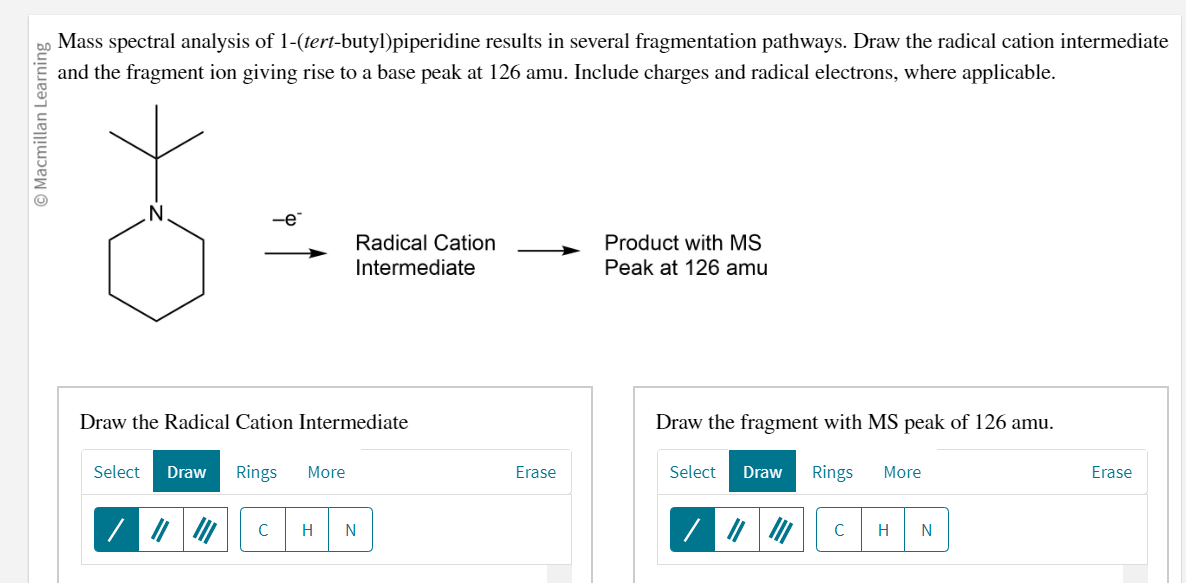
Mass spectral analysis of 1-(tert-butyl)piperidine results in several fragmentation pathways. Draw the radical cation intermediate and the fragment ion giving rise to a base peak at \( 126 \mathrm{amu} \). Include charges and radical electrons, where applicable. \[ \longrightarrow \text { Radical Cation } \longrightarrow \begin{array}{l} -\mathrm{e}^{-} \\ \text {Intermediate } \end{array} \longrightarrow \begin{array}{l} \text { Product with } \mathrm{MS} \\ \text { Peak at } 126 \mathrm{amu} \end{array} \] Draw the fragment with MS peak of \( 126 \mathrm{amu} \).
Expert Answer
Frequency is defined as the number of occurrences of a repeating event per unit of time. For a wave it is the number of waves occurring per unit of ti