Home /
Expert Answers /
Chemistry /
the-lewis-structure-of-carbonate-left-mathrm-co-3-2-right-is-shown-on-the-left-below-pa500
(Solved): The Lewis structure of carbonate \( \left(\mathrm{CO}_{3}^{2}\right) \) is shown on the left below. ...
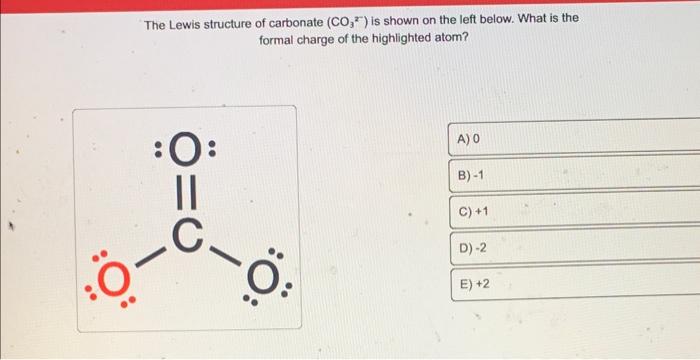
The Lewis structure of carbonate \( \left(\mathrm{CO}_{3}^{2}\right) \) is shown on the left below. What is the formal charge of the highlighted atom?
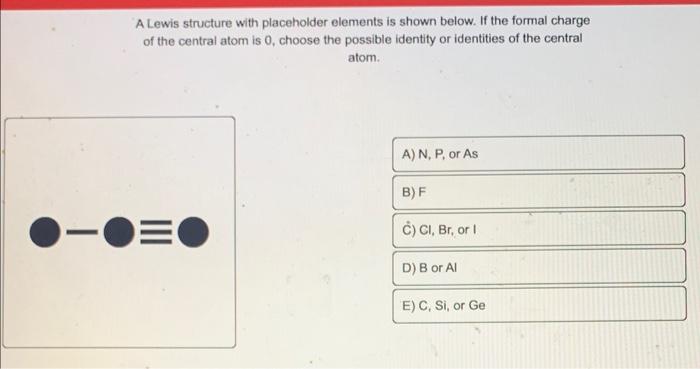
A Lewis structure with placeholder elements is shown below. If the formal charge of the central atom is 0 , choose the possible identify or identities of the central atom.
Expert Answer
Solution: Formal change (FC) is calculated by following equation, FC = V - N - B/2 Where, V = Valence electrons N = Number of lone pair electro