Home /
Expert Answers /
Chemistry /
the-frost-ebsworth-diagrams-of-mathrm-v-in-aqueous-solution-at-mathrm-ph-0-and-at-pa408
(Solved): The Frost-Ebsworth diagrams of \( \mathrm{V} \) in aqueous solution at \( \mathrm{pH}=0 \) and at \ ...
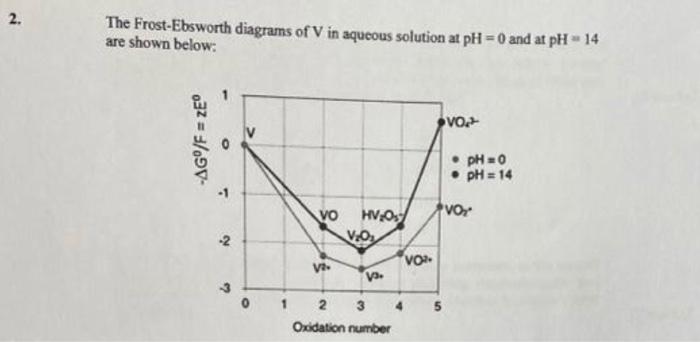
The Frost-Ebsworth diagrams of \( \mathrm{V} \) in aqueous solution at \( \mathrm{pH}=0 \) and at \( \mathrm{pH}=14 \) are shown below:
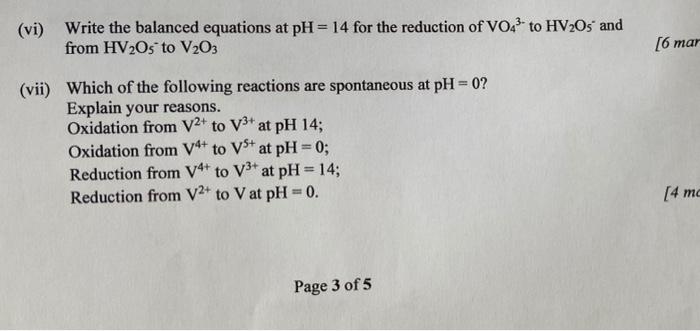
(vi) Write the balanced equations at \( \mathrm{pH}=14 \) for the reduction of \( \mathrm{VO}_{4}{ }^{3-} \) to \( \mathrm{HV}_{2} \mathrm{O}_{5} \) and from \( \mathrm{HV}_{2} \mathrm{O}_{5}^{-} \)to \( \mathrm{V}_{2} \mathrm{O}_{3} \) \( [6 m a \) (vii) Which of the following reactions are spontaneous at \( \mathrm{pH}=0 \) ? Explain your reasons. Oxidation from \( \mathrm{V}^{2+} \) to \( \mathrm{V}^{3+} \) at \( \mathrm{pH} 14 \) Oxidation from \( \mathrm{V}^{4+} \) to \( \mathrm{V}^{5+} \) at \( \mathrm{pH}=0 \); Reduction from \( \mathrm{V}^{4+} \) to \( \mathrm{V}^{3+} \) at \( \mathrm{pH}=14 \); Reduction from \( \mathrm{V}^{2+} \) to \( \mathrm{V} \) at \( \mathrm{pH}=0 \). Page 3 of 5
Expert Answer
Vi) At pH = 14, it is basic solution the balanced equations are as follows. 2 VO43- + 7 H+ + 2e .....> HV2O5- + 3 H2O HV2O5- + 3 H+ +2e .....> V2O3 + 2 H2O vii) In the