Home /
Expert Answers /
Chemistry /
the-following-table-shows-three-compounds-with-their-molecular-mass-their-structures-and-boilin-pa461
(Solved): The following table shows three compounds, with their molecular mass, their structures, and boilin ...
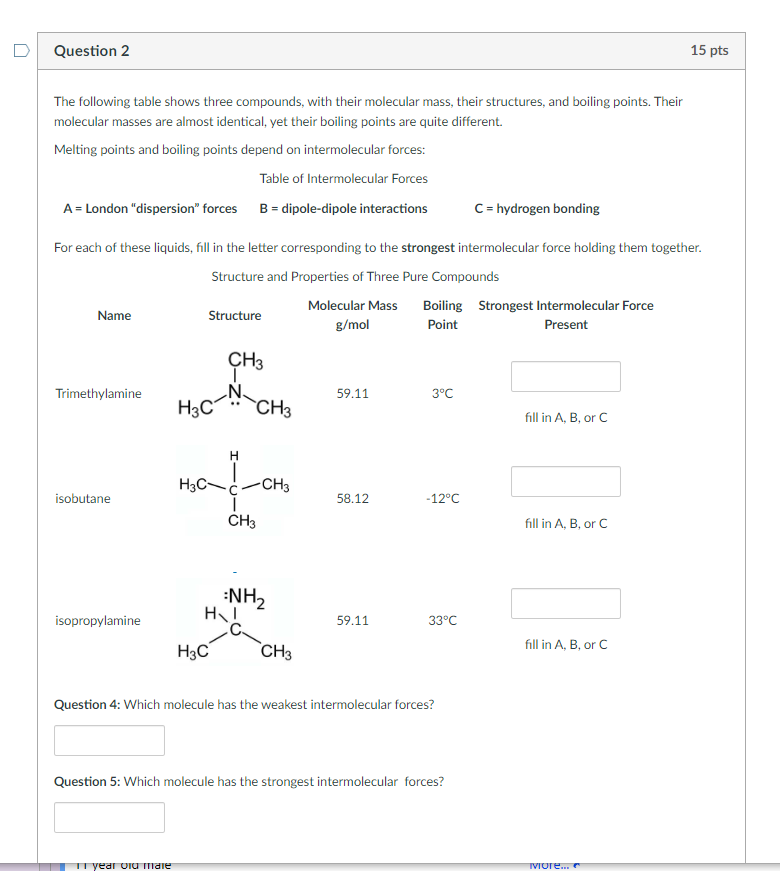
The following table shows three compounds, with their molecular mass, their structures, and boiling points. Their molecular masses are almost identical, yet their boiling points are quite different. Melting points and boiling points depend on intermolecular forces: Table of Intermolecular Forces \( \mathrm{A}= \) London "dispersion" forces \( \mathrm{B}= \) dipole-dipole interactions \( \quad \mathrm{C}= \) hydrogen bonding For each of these liquids, fill in the letter corresponding to the strongest intermolecular force holding them together. Structure and Properties of Three Pure Compounds Question 4: Which molecule has the weakest intermolecular forces? Question 5: Which molecule has the strongest intermolecular forces?