Home /
Expert Answers /
Chemical Engineering /
the-following-isomerization-reaction-occurs-in-the-liquid-phase-a-rightarrow-b-where-pa540
(Solved): : The following isomerization reaction occurs in the liquid phase: \( A \rightarrow B \), where \( ...
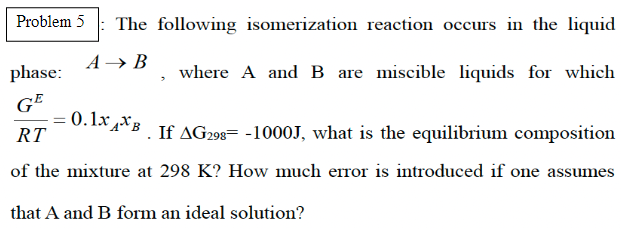
: The following isomerization reaction occurs in the liquid phase: \( A \rightarrow B \), where \( \mathrm{A} \) and \( \mathrm{B} \) are miscible liquids for which \( \frac{G^{E}}{R T}=0.1 x_{A} x_{B} \). If \( \Delta \mathrm{G}_{298}=-1000 \mathrm{~J} \), what is the equilibrium composition of the mixture at \( 298 \mathrm{~K} \) ? How much error is introduced if one assumes that A and B form an ideal solution?