Home /
Expert Answers /
Chemical Engineering /
the-figure-below-represents-a-log-mathrm-c-ph-diagram-from-an-unknown-acid-what-is-the-pa466
(Solved): The figure below represents a \( \log \mathrm{C} \)-pH diagram from an unknown acid. - What is the ...
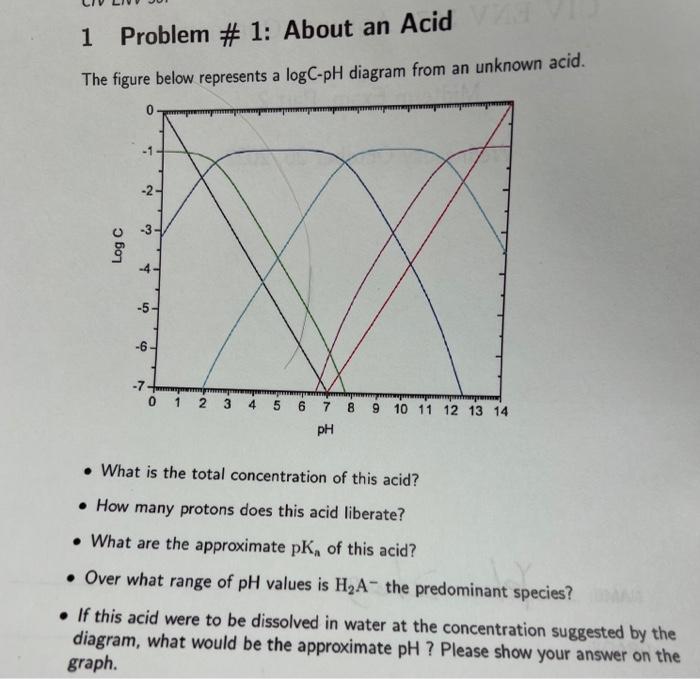
The figure below represents a \( \log \mathrm{C} \)-pH diagram from an unknown acid. - What is the total concentration of this acid? - How many protons does this acid liberate? - What are the approximate \( \mathrm{pK}_{\mathrm{n}} \) of this acid? - Over what range of \( \mathrm{pH} \) values is \( \mathrm{H}_{2} \mathrm{~A}^{-} \)the predominant species? If this acid were to be dissolved in water at the concentration suggested by the diagram, what would be the approximate \( \mathrm{pH} \) ? Please show your answer on the graph.
Expert Answer
The answer is Line 4. REASONS:- Carbonic acid is H2CO3