Home /
Expert Answers /
Chemistry /
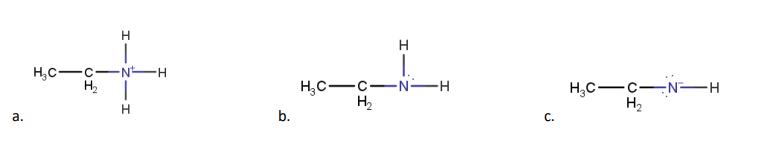
the-ethylammonium-ion-ch3ch2nh3-has-a-pka-of-10-75-which-of-the-following-is-the-dominant-form-pa827
Expert Answer
Solution - CH3CH2NH3+ is the strong conjugate acid of the weak base it means it