Home /
Expert Answers /
Chemistry /
the-decomposition-of-salicylic-acid-to-phenol-and-carbon-dioxide-was-carried-out-at-200-0c-a-t-pa574
(Solved): The decomposition of salicylic acid to phenol and carbon dioxide was carried out at 200.0C, a t ...
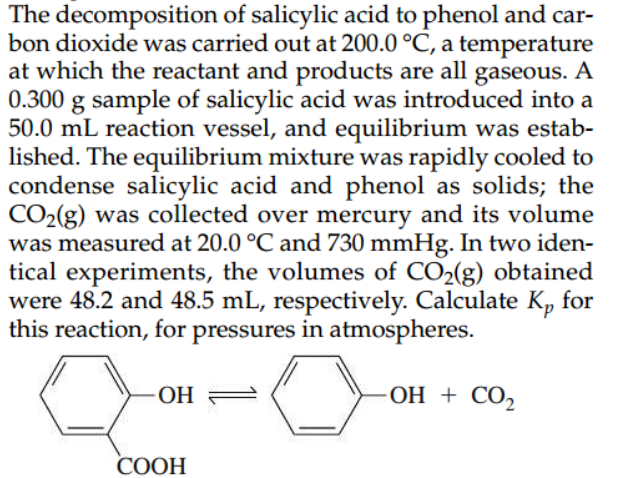
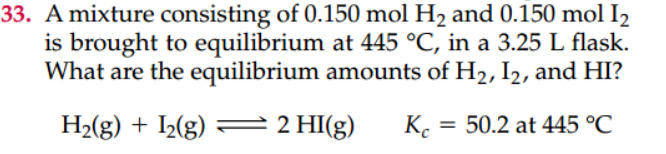
The decomposition of salicylic acid to phenol and carbon dioxide was carried out at , a temperature at which the reactant and products are all gaseous. A sample of salicylic acid was introduced into a reaction vessel, and equilibrium was established. The equilibrium mixture was rapidly cooled to condense salicylic acid and phenol as solids; the was collected over mercury and its volume was measured at and . In two identical experiments, the volumes of obtained were 48.2 and , respectively. Calculate for
3. A mixture consisting of and is brought to equilibrium at , in a 3.25 L flask. What are the equilibrium amounts of , and ?