Home /
Expert Answers /
Chemistry /
the-decomposition-of-5o2cl2-is-first-order-in-5o2cl2-and-has-a-rate-constant-of-1-25-pa443
(Solved): The decomposition of 5O2Cl2 is first order in 5O2Cl2 and has a rate constant of 1.25 ...
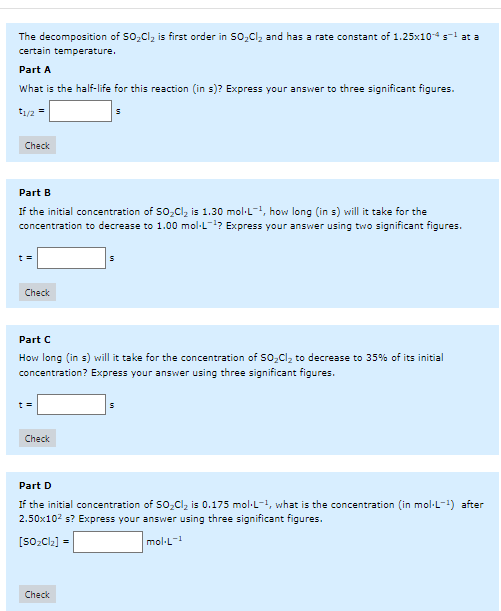
The decomposition of is first order in and has a rate constant of at a certain temperature, Part A What is the half-life for this reaction (in s)? Express your answer to three significant figures. Part B If the initial concentration of is mol. , how long (in ) will it take for the concentration to decrease to ? Express your answer using two significant figures. Part C How long (in s) will it take for the concentration of to decrease to of its initial concentration? Express your answer using three significant figures. Part D If the initial concentration of is mol. , what is the concentration (in mol.L ) after s? Express your answer using three significant figures.
Expert Answer
part-AThe half-life of a first-order reaction given below:t1/2 = 0.693/kwhere k is the rate constant. Plugging in the given rate constant of 1.25 x 10