Home /
Expert Answers /
Chemistry /
the-claisen-condensation-converts-two-molecules-of-an-ester-into-a-beta-keto-ester-the-reac-pa581
(Solved): The Claisen condensation converts two molecules of an ester into a \( \beta \)-keto ester. The reac ...
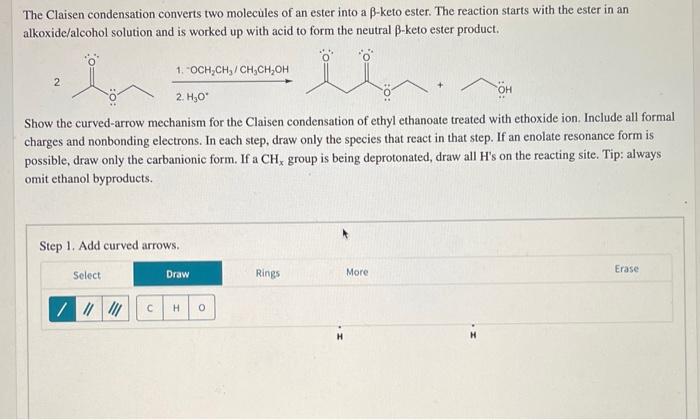
The Claisen condensation converts two molecules of an ester into a \( \beta \)-keto ester. The reaction starts with the ester in an alkoxide/alcohol solution and is worked up with acid to form the neutral \( \beta \)-keto ester product. 2 1. \( { }^{-} \mathrm{OCH}_{2} \mathrm{CH}_{3} / \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{OH} \) Show the curved-arrow mechanism for the Claisen condensation of ethyl ethanoate treated with ethoxide ion. Include all formal charges and nonbonding electrons. In each step, draw only the species that react in that step. If an enolate resonance form is possible, draw only the carbanionic form. If a \( \mathrm{CH}_{x} \) group is being deprotonated, draw all H's on the reacting site. Tip: always omit ethanol byproducts. Step 1. Add curved arrows.
Step 2. Draw the ester-containing intermediate produced from step 1, and draw the next reactant or reagent, if applicable. Add curved arrows and any necessary charges and nonbonding electrons.
Step 3. Draw the ester-containing intermediate produced from step 2, and draw the next reactant or reagent, if applicable. Add curved arrows and any necessary charges and nonbonding electrons.
Step 4. Draw the ester-containing intermediate produced from step 3, and draw the next reactant or reagent, if applicable. Add curved arrows and any necessary charges and nonbonding electrons.
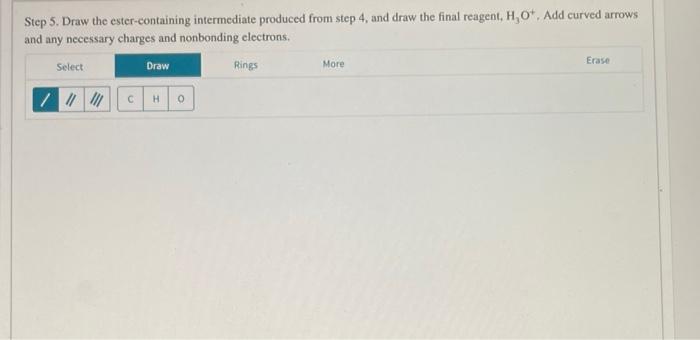
Step 5. Draw the ester-containing intermediate produced from step 4, and draw the final reagent, \( \mathrm{H}_{3} \mathrm{O}^{+} \), Add curved arrows and any necessary charges and nonbonding electrons.
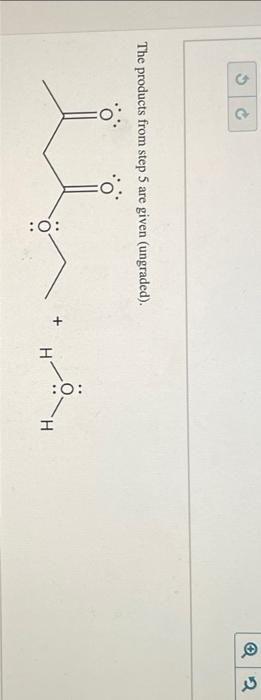
The products from step 5 are given (ungraded).
Expert Answer
Answer:- Step1:- Given reaction is drawn below: Ethoxide is strong base, and it abstracts the acidic proton from substrate that leads to carbanion intermediate. This Claisen condensation reaction starts with proto