Home /
Expert Answers /
Chemistry /
the-central-oxygen-atom-in-mathrm-o-3-calculate-the-formal-charge-on-the-indicated-atom-i-pa135
(Solved): the central oxygen atom in \( \mathrm{O}_{3} \) Calculate the formal charge on the indicated atom i ...
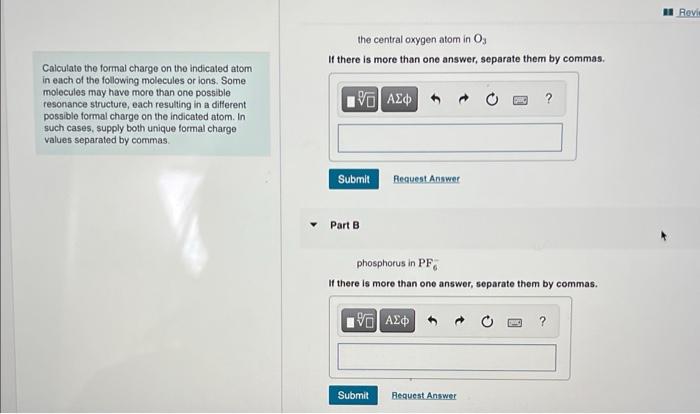
the central oxygen atom in \( \mathrm{O}_{3} \) Calculate the formal charge on the indicated atom in each of the following molecules or ions. Some molecules may have more than one possible resonance structure, each resulting in a different possible formal charge on the indicated atom. In such cases, supply both unique formal charge values separated by commas. Part B phosphorus in \( \mathrm{PF}_{6} \) If there is more than one answer, separate them by commas.
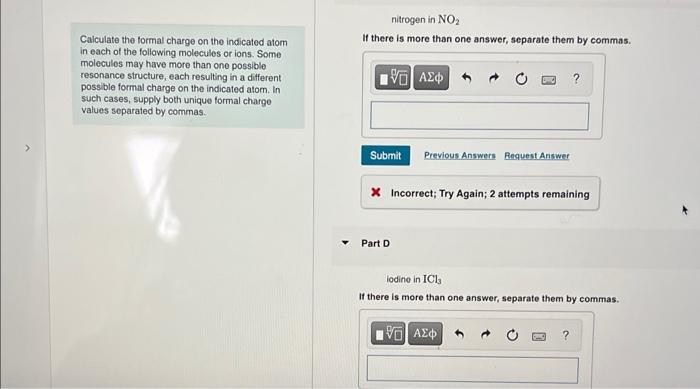
nitrogen in \( \mathrm{NO}_{2} \) Calculate the formal charge on the indicated atom If there is more than one answer, separate them by commas. in each of the following molecules or ions. Some molecules may have more than one possible resonance structure, each resulting in a different possible formal charge on the indicated atom. In such cases, supply both unique formal charge values separated by commas. * Incorrect; Try Again; 2 attempts remaining Part D lodine in \( \mathrm{ICl}_{3} \) If there is more than one answer, separate them by commas.
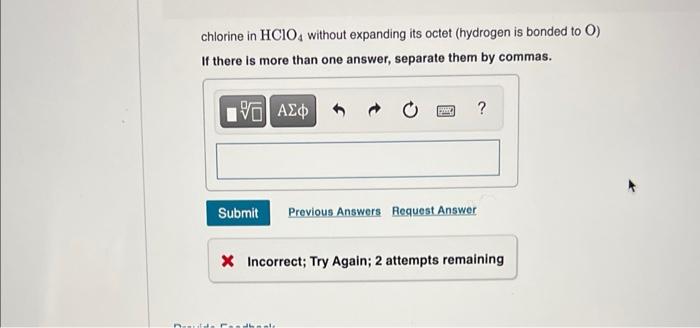
chlorine in \( \mathrm{HClO}_{4} \) without expanding its octet (hydrogen is bonded to \( \mathrm{O} \) ) If there is more than one answer, separate them by commas. * Incorrect; Try Again; 2 attempts remaining
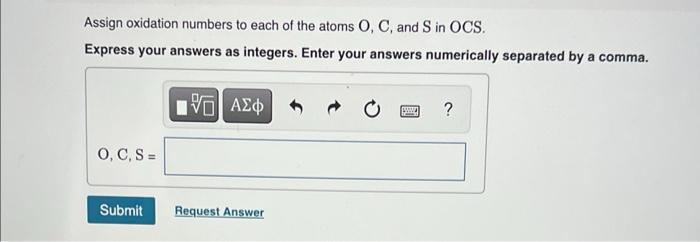
Assign oxidation numbers to each of the atoms \( \mathrm{O}, \mathrm{C} \), and \( \mathrm{S} \) in \( \mathrm{OCS} \). Express your answers as integers. Enter your answers numerically separated by a comma.
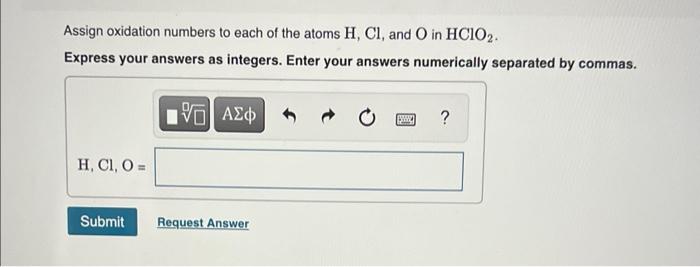
Assign oxidation numbers to each of the atoms \( \mathrm{H}, \mathrm{Cl} \), and \( \mathrm{O} \) in \( \mathrm{HClO}_{2} \). Express your answers as integers. Enter your answers numerically separated by commas.