Home /
Expert Answers /
Chemistry /
the-blood-buffering-bicarbonate-equilibrium-is-shown-below-mathrm-co-2-mathrm-h-2-mat-pa134
(Solved): The blood buffering bicarbonate equilibrium is shown below: \( \mathrm{CO}_{2}+\mathrm{H}_{2} \mat ...
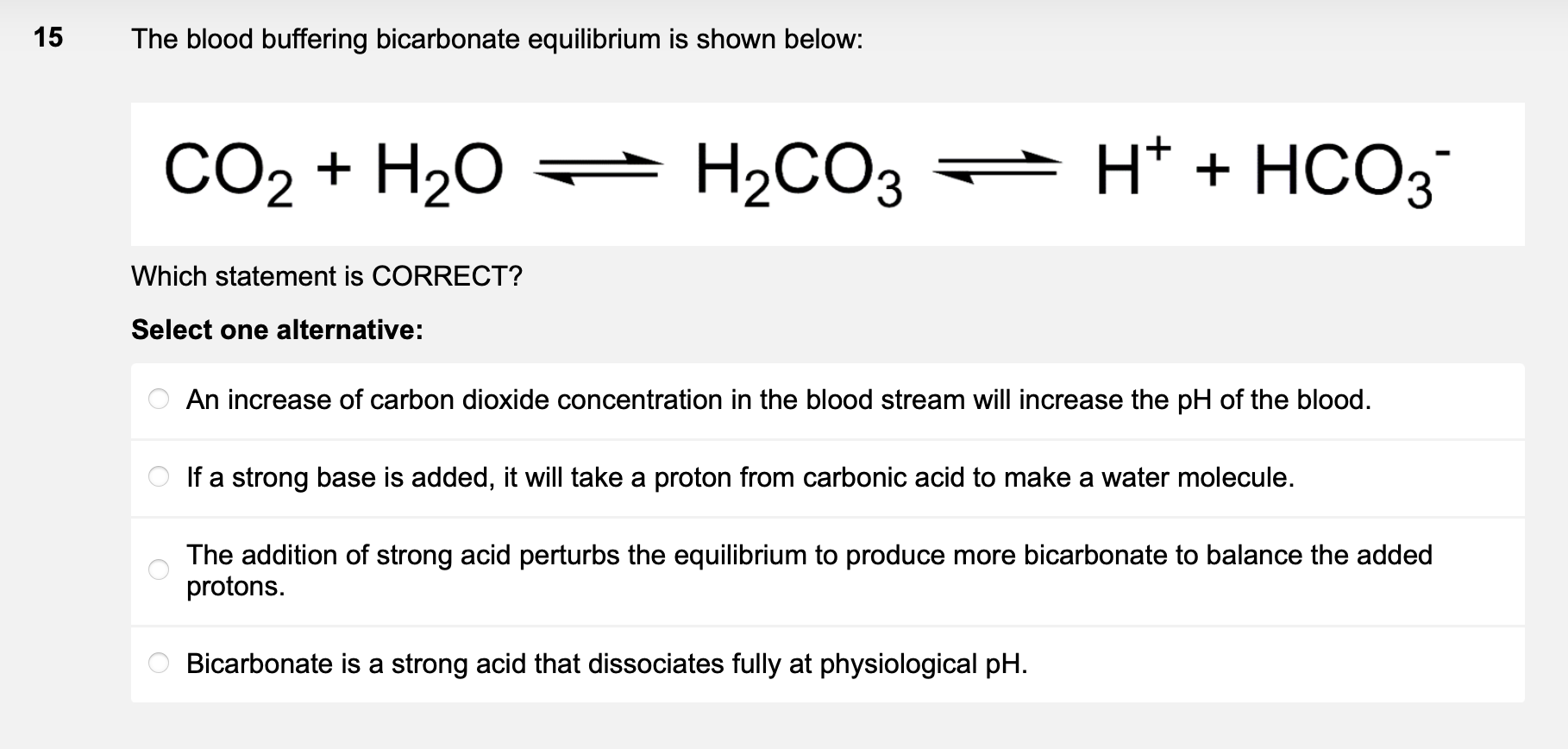
The blood buffering bicarbonate equilibrium is shown below: \( \mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O} \rightleftharpoons \mathrm{H}_{2} \mathrm{CO}_{3} \rightleftharpoons \mathrm{H}^{+}+\mathrm{HCO}_{3}^{-} \) Which statement is CORRECT? Select one alternative: An increase of carbon dioxide concentration in the blood stream will increase the \( \mathrm{pH} \) of the blood. If a strong base is added, it will take a proton from carbonic acid to make a water molecule. The addition of strong acid perturbs the equilibrium to produce more bicarbonate to balance the added protons. Bicarbonate is a strong acid that dissociates fully at physiological \( \mathrm{pH} \).
Which statement is CORRECT? Select one alternative: In their unfolded state, proteins display many conformations. The formation of new weak interactions in protein folding increases the overall Gibbs energy. Entropy is a measure of the disorder or chaos of molecules in a system. The hydrophobic effect is important in protein folding but not in any other cellular processes.
Expert Answer
Answer (15) : (b) If a strong acid is added , it will take a proton from carbonic acid to form water. A base generally contains OH- ions which react with proton H+ of carbonic acid to form water (H2O). Option (a) Not correct. Increas