Home /
Expert Answers /
Chemistry /
the-balanced-equation-for-the-neutralization-reaction-of-aqueous-hso4-with-aqueous-koh-is-shown-pa974
(Solved): The balanced equation for the neutralization reaction of aqueous HSO4 with aqueous KOH is shown. ...
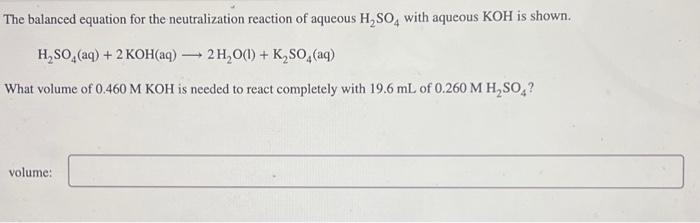
The balanced equation for the neutralization reaction of aqueous H₂SO4 with aqueous KOH is shown. H₂SO4 (aq) + 2 KOH(aq) → 2 H₂O(1) + K₂SO4 (aq) What volume of 0.460 M KOH is needed to react completely with 19.6 mL of 0.260 M H₂SO4? volume:
The balanced equation for the neutralization reaction of aqueous with aqueous is shown. What volume of is needed to react completely with of ?