Home /
Expert Answers /
Chemistry /
the-activation-energy-of-the-reaction-between-persulfate-and-iodide-ions-during-the-reaction-of-t-pa895
(Solved): THE ACTIVATION ENERGY OF THE REACTION BETWEEN PERSULFATE AND IODIDE IONS, During the reaction of t ...
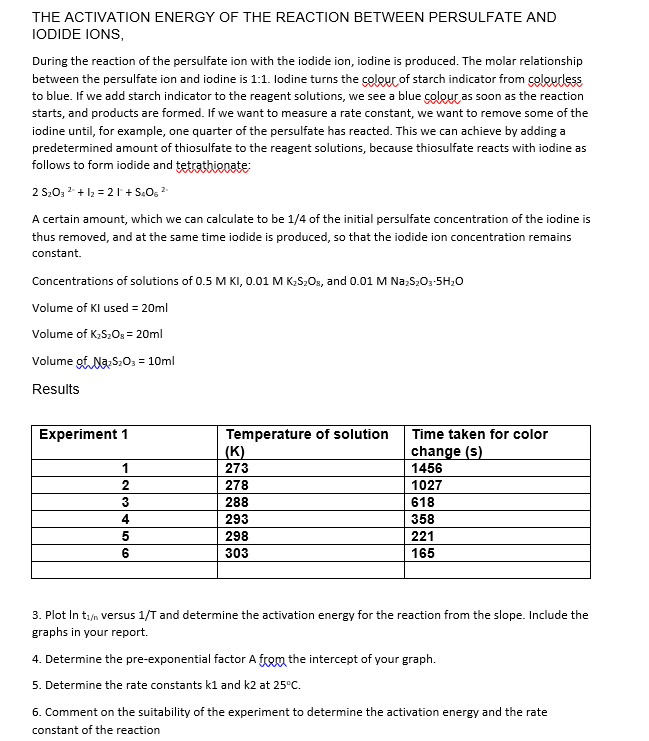
THE ACTIVATION ENERGY OF THE REACTION BETWEEN PERSULFATE AND IODIDE IONS, During the reaction of the persulfate ion with the iodide ion, iodine is produced. The molar relationship between the persulfate ion and iodine is 1:1. lodine turns the colouc of starch indicator from colourless to blue. If we add starch indicator to the reagent solutions, we see a blue colour as soon as the reaction starts, and products are formed. If we want to measure a rate constant, we want to remove some of the iodine until, for example, one quarter of the persulfate has reacted. This we can achieve by adding a predetermined amount of thiosulfate to the reagent solutions, because thiosulfate reacts with iodine as follows to form iodide and tetrathionate: \[ 2 \mathrm{~S}_{2} \mathrm{O}_{3}{ }^{2-}+\mathrm{I}_{2}=2 \mathrm{I}^{2}+\mathrm{S}_{4} \mathrm{O}_{6}{ }^{2} \] A certain amount, which we can calculate to be \( 1 / 4 \) of the initial persulfate concentration of the iodine is thus removed, and at the same time iodide is produced, so that the iodide ion concentration remains constant. Concentrations of solutions of \( 0.5 \mathrm{M} \mathrm{KI}, 0.01 \mathrm{M} \mathrm{K}_{2} \mathrm{~S}_{2} \mathrm{O}_{8} \), and \( 0.01 \mathrm{M} \mathrm{Na}_{2} \mathrm{~S}_{2} \mathrm{O}_{3} \cdot 5 \mathrm{H}_{2} \mathrm{O} \) Volume of \( \mathrm{Kl} \) used \( =20 \mathrm{ml} \) Volume of \( \mathrm{K}_{2} \mathrm{~S}_{2} \mathrm{O}_{\mathrm{s}}=20 \mathrm{ml} \) Volume of \( \mathrm{Na}_{2} \mathrm{~S}_{2} \mathrm{O}_{3}=10 \mathrm{ml} \) Results 3. Plot In \( t_{1 / n} \) versus \( 1 / T \) and determine the activation energy for the reaction from the slope. Include the graphs in your report. 4. Determine the pre-exponential factor A from the intercept of your graph. 5. Determine the rate constants \( \mathrm{k} 1 \) and \( \mathrm{k} 2 \) at \( 25^{\circ} \mathrm{C} \). 6. Comment on the suitability of the experiment to determine the activation energy and the rate constant of the reaction
Expert Answer
3. Data table: Experiment T (K) 1/T (K-1) t1/n (s-1) ln t1/n 1 273 0.003663 0.0007 -7.2834 2 278 0.003597 0.0010 -6.9344 3 288 0.003472 0.0016 -6