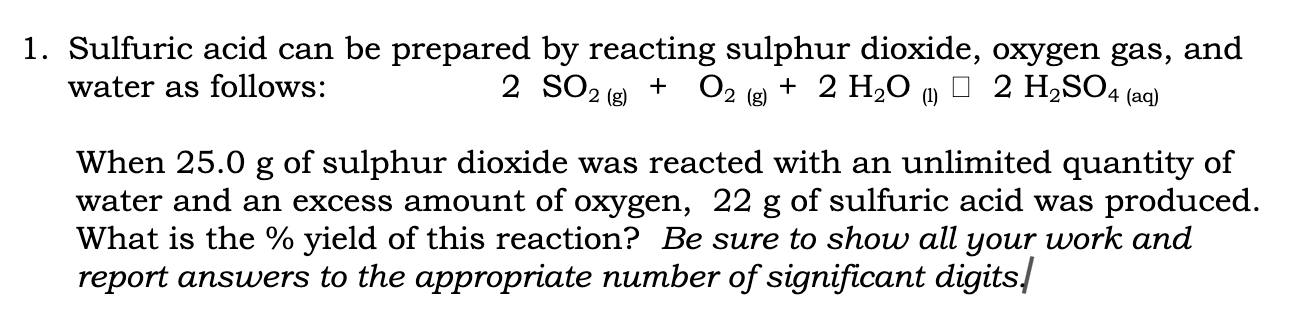Home /
Expert Answers /
Chemistry /
sulfuric-acid-can-be-prepared-by-reacting-sulphur-dioxide-oxygen-gas-and-water-as-follows-2-pa111
(Solved): Sulfuric acid can be prepared by reacting sulphur dioxide, oxygen gas, and water as follows: \[ 2 ...
Sulfuric acid can be prepared by reacting sulphur dioxide, oxygen gas, and water as follows: \[ 2 \mathrm{SO}_{2(\mathrm{~g})}+\mathrm{O}_{2(\mathrm{~g})}+2 \mathrm{H}_{2} \mathrm{O}{ }_{(\mathrm{l})} \square 2 \mathrm{H}_{2} \mathrm{SO}_{4(\mathrm{aq})} \] When \( 25.0 \) g of sulphur dioxide was reacted with an unlimited quantity of water and an excess amount of oxygen, \( 22 \mathrm{~g} \) of sulfuric acid was produced. What is the \% yield of this reaction? Be sure to show all your work and report answers to the appropriate number of significant digits.