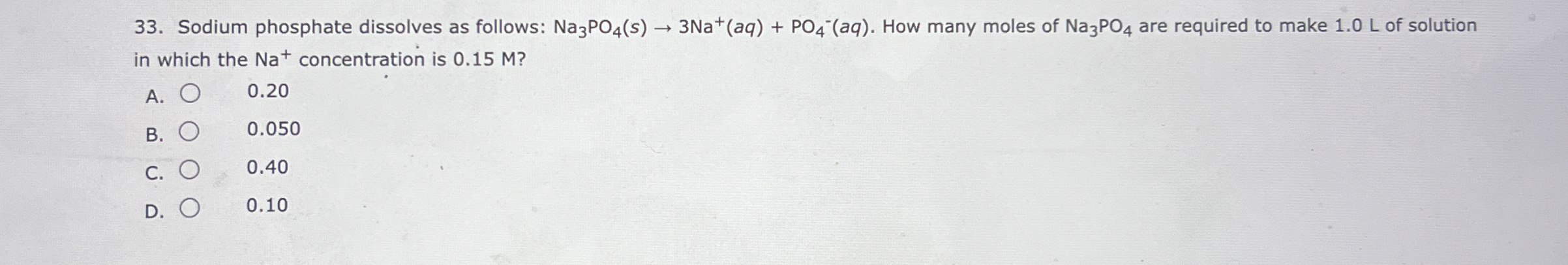Home /
Expert Answers /
Chemistry /
sodium-phosphate-dissolves-as-follows-na-3-po-4-s-gt-3na-aq-po-4-aq-how-many-mole-pa907
(Solved): Sodium phosphate dissolves as follows: Na_(3)PO_(4)(s)->3Na^(+)(aq)+PO_(4)^(-)(aq). How many mole ...
Sodium phosphate dissolves as follows:
Na_(3)PO_(4)(s)->3Na^(+)(aq)+PO_(4)^(-)(aq). How many moles of
Na_(3)PO_(4)are required to make 1.0 L of solution in which the
Na^(+)concentration is 0.15 M ? A.
◻0.20 B.
◻0.050 C.
◻0.40 D.
◻0.10