Home /
Expert Answers /
Chemistry /
sodium-borohydride-reacts-with-water-as-follows-nabh4-s-4-h2o-1-nab-oh-4-aq-4-h2-g-pa835
(Solved): Sodium borohydride reacts with water as follows: NaBH4 (s) + 4 H2O (1) --> NaB(OH)4 (aq) + 4 H2 (g) ...
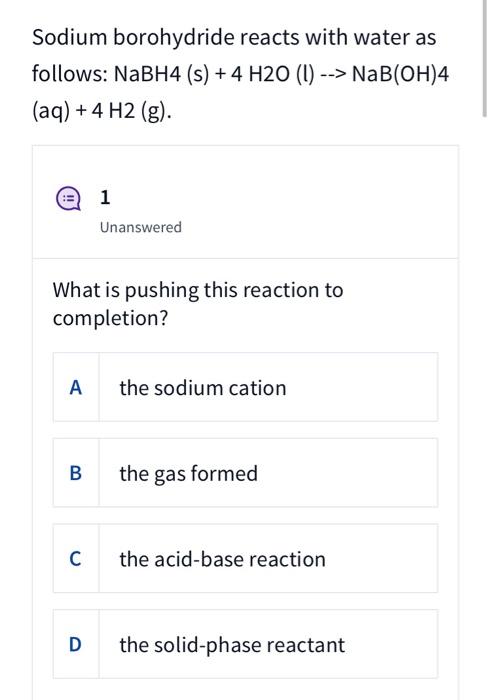
Sodium borohydride reacts with water as follows: NaBH4 (s) + 4 H2O (1) --> NaB(OH)4 (aq) + 4 H2 (g). 1 Unanswered What is pushing this reaction to completion? A the sodium cation B the gas formed C the acid-base reaction D the solid-phase reactant