Home /
Expert Answers /
Physics /
salting-out-dna-con-consmer-a-negatively-charged-segment-of-double-helical-dna-in-anionic-solat-pa326
(Solved): Salting Out DNA CON CONSMER a (negatively charged) segment of double-helical DNA in anionic solat ...
Salting Out DNA CON “CONSMER a (negatively charged) segment of double-helical DNA in anionic solation consisting of equal nombs of positive and negative ions, illustrated in the cartoon bedow (talion from Phillips, et al. Physical Biology of the Cell.) Fre 14 i 1. Why don't the positive ione attach all along the length of the DNA until the DNA-is dectrically untral? What, if anything, opposes that process? Adapted from the work of R.P. Redish and collaboradors Pablished at compadre.org/nomph and thened under the CC BY-NC-SA 5. Now let's an in a hit and look at the struction of a segment of DNA more chmely. One way to denature DNA (L., to heak the double helis into two single strands) is by changing the one strength of the solution in which it exists. DNA dentures pote easily when moved from a solution of considerable nic strength (salt water, fe example) to pare dhatilled water. Why might it be much carte desture DNA in pie water than it is in salt water? 1. The positive chand of was surrounding the negatively charged DNA olsa datan Ap, called the Debye ngth" Just outside the Debye length, the electric fiel magnitode is amaller than it would be if not for the positive ionic clond. Salting Out" is the proces by which pieces of DNA hump together and precipitate out of solution. Does the presence of the lona in solution make it harder or cater for this chumping to occur (compared to the case of pure water) 1. Discus with your group how the Delive length would change with 1) the temperature and 2) the salt concentration, and give an argument as to why these dependencies are conceptually plausible Ap- 4. Using your ans bom parts 2 and 3, decide whether the salting out of DNA from solution is more likely to over the temperature increases in deces. Decide whether the "salting out of DNA me likely to occur as the contration of all geta larger or smaller. Adapted from the work of EF. Hedish and collators. Published at compadre.org/gh and bened under the CC BY-NC-SA

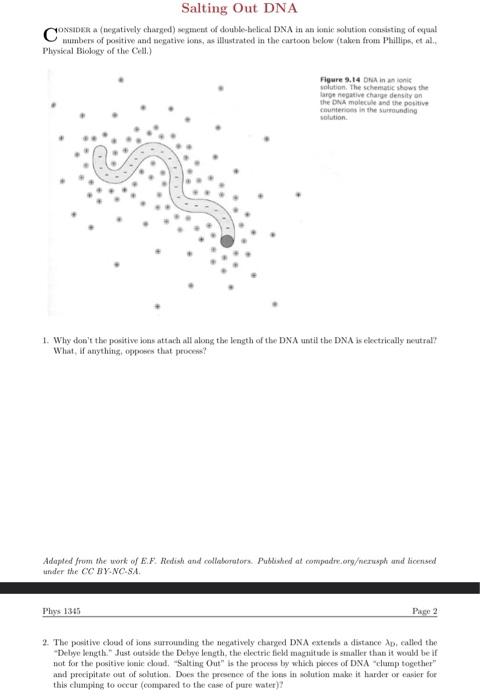

Salting Out DNA wedur dis de ar-Nexi. aresus Dhes
Cossabis a (acyatively charged) segraent of double-belical DNA in an knie solution coessisting of expaal numbers of positive and negative ions, as illustrated in the cartoon below (talora from Phillips, et al.. Physical Biology of the Celi, ) Figure Dha in as ionis solution. The schematic skows the laroe negative eharye density on the DAs molecule ind the positive counterions in the survondine volution. 1. Why doe't the positive ioas attach all along the leagth of the DNA until tbe DNA is elkctrically neutrall? What, if anything, oppoens that pevoens? under the . Phys 1345 Page 2 2. The positive eloul of jons sarroundiag the negatively charged DNA exteals a distance , callied the "Delye kngth." Jist outside the Debye length, the electric field magnitude is smaller thas is woobl be if not for the positive ineir cloul. "Saltiag Out" is the process by which pores of DNA "clumap together" and precipitate out of solutioe. Does the persence of the joes in aolution male it larder of ensier for this clumping to occur (comparext to the case of pure water)?
3. Discuss with your group how the Debye length would change with 1) the temperature and 2) the salt concentration, and give an argument as to why these dependencies are conceptually plausible. 4. Using your answers from parts 2 and 3 , decide whether the "salting out" of DNA from solution is more likely to occur as the temperature increases or decreases. Decide whether the "salting out" of DNA is more likely to occur as the concentration of salt gets larger or smaller. Adapted from the work of E.F. Redish and collaborators. Published at compadre,org/nerusph and licensed under the CC BY-NC-SA. Phys 1345 Page 3 5. Now let's zoom in a bit and look at the structure of a segment of DNA more closely. One way to denature DNA (i.e., to break the double helix into two single strands) is by changing the ionic strength of the solution in which it exists. DNA denatures quite easily when moved from a solution of considerable ionic strength (salt water, for example) to pure distilled water. Why might it be much easier to denature DNA in pure water than it is in salt water?