Home /
Expert Answers /
Mechanical Engineering /
required-information-the-refrigerant-134a-is-in-vapor-state-at-0-9-mathrm-mpa-and-70-c-pa332
(Solved): Required information The refrigerant-134a is in vapor state at \( 0.9 \mathrm{MPa} \) and \( 70^{\c ...
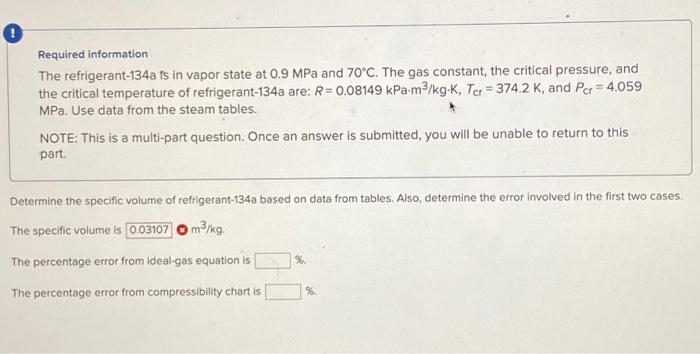
Required information The refrigerant-134a is in vapor state at \( 0.9 \mathrm{MPa} \) and \( 70^{\circ} \mathrm{C} \). The gas constant, the critical pressure, and the critical temperature of refrigerant-134a are: \( R=0.08149 \mathrm{kPa} \cdot \mathrm{m}^{3} / \mathrm{kg} \cdot \mathrm{K}, T_{\mathrm{cr}}=374.2 \mathrm{~K} \), and \( P_{\mathrm{cr}}=4.059 \) MPa. Use data from the steam tables. NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. Determine the specific volume of refrigerant-134a based on data from tables. Also, determine the error involved in the first two cases. The specific volume is The percentage error from ideal-gas equation is The percentage error from compressibility chart is