Home /
Expert Answers /
Chemistry /
rank-the-following-elements-in-order-of-decreasing-electronegativity-from-most-electronegative-pa438
(Solved): Rank the following elements in order of decreasing electronegativity (from most electronegative ...
Rank the following elements in order of decreasing electronegativity (from most electronegative to least electronegative): View Available Hint(s) \[ \begin{array}{l} \mathrm{Br}>\mathrm{Te}>\mathrm{Se}>\mathrm{Sb} \\ \mathrm{Br}>\mathrm{Se}>\mathrm{Te}>\mathrm{Sb} \\ \mathrm{Sb}>\mathrm{Te}>\mathrm{Se}>\mathrm{Br} \\ \mathrm{Se}>\mathrm{Br}>\mathrm{Sb}>\mathrm{Te} \end{array} \]
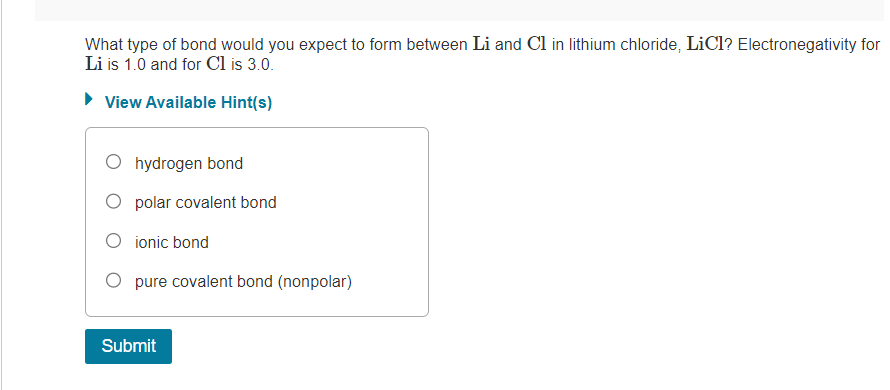
What type of bond would you expect to form between \( \mathrm{Li} \) and \( \mathrm{Cl} \) in lithium chloride, \( \mathrm{LiCl} \) ? Electronegativity for \( \mathrm{Li} \) is \( 1.0 \) and for \( \mathrm{Cl} \) is \( 3.0 \). View Available Hint(s) hydrogen bond polar covalent bond ionic bond pure covalent bond (nonpolar)
Expert Answer
(1) Electronegativity of elements Br = 2.8 Te = 2.1 Se